March 17, 2015 — The National Oncologic PET Registry (NOPR) Working Group submitted a formal request to the Centers for Medicare and Medicaid Services (CMS), to end the prospective data collection requirements under Coverage with Evidence Development (CED) for use of NaF-18 PET (CAG-00065R) imaging in intended patient management. CMS has initiated its review of this request.
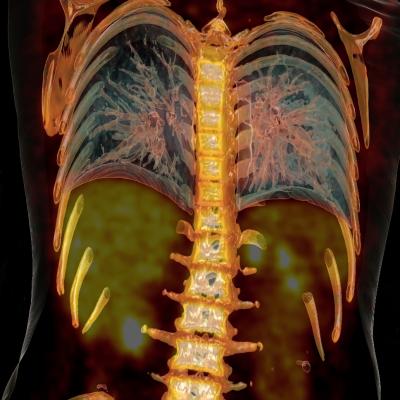
Positron emission tomography (PET) is a minimally invasive imaging procedure used to assess metabolic activity and perfusion in various organs or tissues. Images are obtained by processing of emissions from positron-emitting radioisotopes that are usually administered intravenously. Sodium fluoride F-18 (NaF-18) is a radioisotope commonly used in oncologic PET imaging procedures to detect bone metastasis in cancer and is the focus of this national coverage analysis.
The rate of NaF-19 uptake provides information on the tissues being studied. NaF-18 PET evaluation can indicate the probable presence or absence of bone metastasis based upon observed differences in biologic activity of adjacent tissues.
Section 220.6.19 of the National Coverage Determination (NCD) Manual establishes the requirement for prospective data collection under CED for NaF-18 PET for bone metastasis in cancer.
At this time, CMS is soliciting comments on the NOPR's request. The public comment period will be open for 30 days, until April 16, 2015. PET facilities participating in the NOPR are encouraged to provide comments (which can be entered directly online on the CMS website at this link). Additionally, PET facilities are encouraged to ask referring physicians who frequently use NaF-18 PET in the care of their patients to submit comments to CMS indicating how NaF-18 PET has helped with patient management. Comments that cite scientific evidence (including unpublished data), including the full range of uses of NaF-18 PET in oncology and the uses of NaF-18 PET in benign bone and joint diseases, will be particularly valuable to CMS.
For more information: www.cms.gov


 February 26, 2026
February 26, 2026 









