December 9, 2008 – The FDA issued 510(k) approval for market clearance of nordicICE BOLD and DTI Modules developed by NordicNeuroLab (NNL), applications optimizing workflow associated with analyzing and combining Diffusion Tensor Imaging and BOLD fMRI data.
The newest version of NordicImagingLab’s main software framework dedicated to functional MR imaging methodologies (BOLD, DTI, Perfusion) is nordicICE v2.3. nordicICE BOLD and DTI analysis modules integrate with the NNL Hardware System and nordicAktiva, the paradigm and workflow software which completes the nordic fMRI solution.
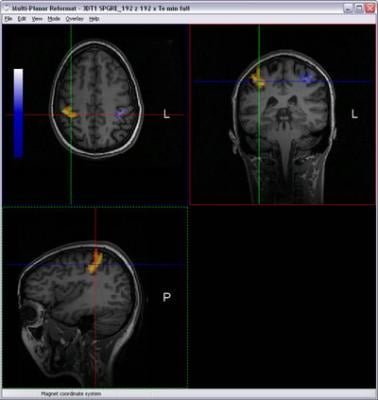
One of the new features in nordicICE v2.3 is the BOLD and DTI Wizard - dedicated to simplifying and optimizing the workflow associated with analyzing and combining Diffusion Tensor Imaging and BOLD fMRI data. This wizard provides an intuitive and easy-to-use step-by-step interface that guides the user through the process of loading, analyzing and visualizing multimodality imaging data. The new integrated Multi-Planar Reconstruction and 3D visualization interface offers unique tools for combining functional activation maps and white matter fiber tracts on a structural image volume. The resulting datasets (both fiber structures and activation maps) can be readily exported to various neuronavigation and treatment planning systems.
This Windows-based application is easy to use and can be readily integrated into the clinical workflow in any hospital or institution. At the same time, nordicICE offers the research oriented user the possibility to take advantage of the myriad of advanced possibilities offered by this versatile, high-performance application. nordicICE v2.3 runs on Windows 2000, XP or Vista platforms.
For more information: www.nordicneurolab.com


 February 13, 2026
February 13, 2026 









