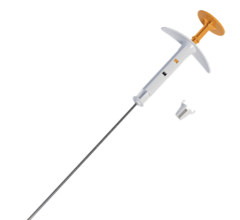
May 29, 2012 — Cianna Medical, maker of the SAVI breast brachytherapy applicator, recently announced the launch of BEST (Brachytherapy Efficacy, Safety and Treatment) Forum, a new educational resource for physicians to obtain the latest research in breast brachytherapy and gain insights into best practices and available technology.
Both online and in person, BEST Forum brings together thought leaders in the field of breast cancer care to discuss their extensive experiences and research in brachytherapy, treatment and clinical outcomes. Breast brachytherapy is a form of accelerated partial breast irradiation (APBI), a shortened course of radiation therapy for early-stage breast cancer patients following lumpectomy surgery.
At the BEST Forum website, which launched in early May, physicians can view presentations from brachytherapy experts, as well as download white papers, presentation slides and supporting clinical data, and register for future presentations.
"We're excited to introduce this unique resource, as it enables physicians interested in breast brachytherapy to learn from their colleagues' experiences while providing them with the tools to educate referring physicians in their own communities about the benefits of this accelerated treatment option," said Jill Anderson, president and CEO of Cianna Medical.
Volume 1 of BEST Forum features radiation oncologist Robert Kuske, M.D., FAACE, from Arizona Breast Cancer Specialists in Scottsdale, Ariz. In Practical Application of Patient Selection Criteria, Kuske discusses the origin and common misconceptions regarding current patient selection guidelines from the American Society of Radiation of Oncology (ASTRO) for the use of APBI.
In addition to discussing his own experiences, Kuske reviews emerging data on APBI, focused on controversial subsets such as women with estrogen receptor (ER) negative tumors, ductal carcinoma in situ (DCIS) and younger age (<60 years). Kuske also explains how strut-based brachytherapy can expand the ability to treat eligible patients where anatomical restrictions prevent the use of other forms of breast brachytherapy.
Volume 2, Lower Toxicities with Strut-based Brachytherapy: 4-year Results, is scheduled to be released in July. Catheryn Yashar, M.D., a radiation oncologist at UC San Diego Moores Cancer Center in La Jolla, Calif., will provide an in-depth analysis on published data from the SAVI Collaborative Research Group, including acute and late toxicity, local recurrence rates and how strut-based brachytherapy can expand patient eligibility for APBI.
The published data has been presented at several medical conferences in 2012, including the American College of Radiation Oncology (ACRO), the American Society of Breast Disease (ASBD) and the American Brachytherapy Society (ABS) World Congress of Brachytherapy.
For more information: www.thebestforum.com


 October 01, 2023
October 01, 2023