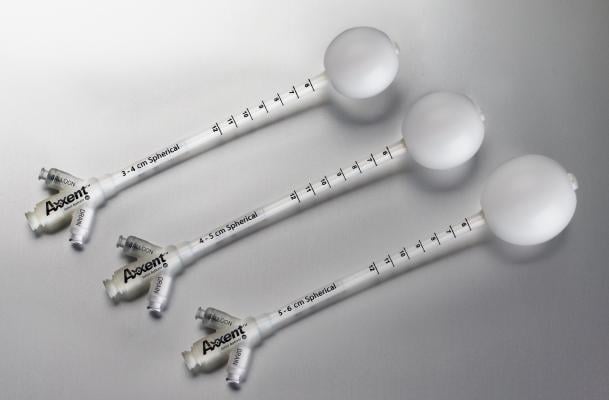
August 30, 2017 — iCAD Inc. announced that the company’s Xoft Axxent balloon applicators have received approval from the China Food & Drug Administration (CFDA) for the treatment of early-stage breast cancer. The Xoft Axxent balloon applicators are a key component of the Xoft Axxent Electronic Brachytherapy (eBx) System, which is used to perform intraoperative radiation therapy (IORT) in early-stage breast cancer patients who meet specific selection criteria.
With this CFDA approval, the complete suite of Xoft System products is now available to clinicians and patients in China.
IORT with the Xoft System allows radiation oncologists and breast surgeons to work together to deliver a full course of radiation to the tumor site at the time of lumpectomy. The balloon applicator is used to deliver a single, precise dose of radiation to the lumpectomy cavity, directly targeting cancer cells while minimizing exposure to healthy surrounding tissue. In the procedure, the Xoft System’s miniaturized X-ray source is inserted into the balloon applicator, providing a channel to deliver high-dose rate, low-energy radiation treatment. The balloon applicator is available in multiple sizes and shapes, can be filled with different volumes of saline to best fit the contour of the surgical cavity, and allows delivery of a more conformal radiation dose.
“The Xoft Axxent balloon applicators offer an attractive option for physicians and patients who desire improved flexibility, precision and personalization in the targeted treatment of early-stage breast cancer,” said Liu Yu, COO of Chindex Medical Limited, the distributor of iCAD’s Xoft System in China. “We are pleased to continue working with iCAD in offering our customers access to the most advanced cancer treatments, including the complete portfolio of Xoft System products, in the years ahead.”
The Xoft System is U.S. Food and Drug Administration (FDA)-cleared for the treatment of cancer anywhere in the body, including early-stage breast cancer, non-melanoma skin cancer and gynecological cancers.
For more information: www.xoftinc.com


 February 18, 2026
February 18, 2026 









