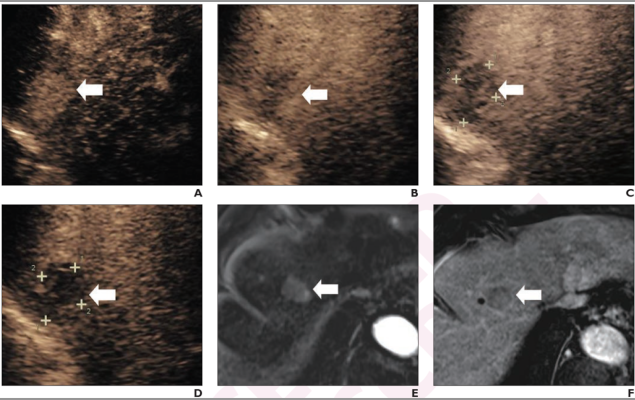
71-year-old woman at high-risk for HCC due to chronic hepatitis B. Patient underwent CEUS using perfluorobutane. (A) Arterial-phase CEUS image (12 seconds after injection) shows 25-mm segment-8 lesion with nonrim hyperenhancement (arrow). (B) CEUS image in portal-venous phase (48 seconds) shows corresponding early washout (arrow). (C) CEUS image in late phase (2 minutes) shows corresponding late and mild washout (arrow). (D) CEUS image in Kupffer phase (15 minutes) shows marked Kupffer defect (arrow). Patient also underwent evaluation by dynamic contrast-enhanced MRI using gadoxetate disodium. (E) Axial arterial-phase image shows 23-mm segment-8 lesion with nonrim arterial phase hyperenhancement (arrow). (F) Axial portal-venous phase image shows corresponding nonperipheral washout (arrow). Observation was classified as LR-M by modified CEUS criteria using perfluorobutane, and as LR-5 by CT/MRI LI-RADS v2018. Pathologic diagnosis based on surgical resection was intrahepatic cholangiocarcinoma.
November 17, 2022 — According to an accepted manuscript published in ARRS’ American Journal of Roentgenology (AJR), diagnostic performance of LR-5 for hepatocellular carcinoma (HCC) diagnosis was not significantly different between modified contrast-enhanced ultrasound (CEUS) using perfluorobutane and CT/MRI LI-RADS version 2018.
“The findings support the application of modified CEUS criteria using perfluorobutane for diagnosing HCC in high-risk patients,” wrote Jianhua Zhou, MD, PhD, of Sun Yat-Sen University Cancer Center’s State Key Laboratory of Oncology in Guangzhou, South China.
In this AJR accepted manuscript, 171 patients (140 men, 31 women; mean age, 54 years) at high risk for HCC with a pathologically confirmed liver observation were evaluated by both CEUS using perfluorobutane and contrast-enhanced CT or MRI, between March 2020 and May 2021. A matching algorithm was used to select 2 patients with HCC for each patient with a non-HCC lesion. Two readers evaluated observations using proposed modifications to CEUS LI-RADS version 2017 that classify certain observations as LR-5—rather than LR-4 or LR-M, based on presence of defect post-perfluorobutane administration.
Ultimately, modified CEUS criteria using perfluorobutane and CT/MRI LI-RADS v2018 showed no significant difference in sensitivity (92.1% vs. 89.5%), specificity (87.9% vs. 84.2%), or accuracy (90.6% vs. 87.7%) of LR-5 for HCC. All observations assigned LR-4 (n=5) or LR-M (n=6) by CT/MRI LI-RADS v2018 but LR-5 by modified CEUS were HCC.
“Modified CEUS criteria performed similarly as CT/MRI LI-RADS v2018 and additional HCCs in a subset of observations assigned LR-4 or LR-M by CT/MRI LIRADS v2018,” the authors of this AJR accepted manuscript reiterated.
For more information: www.arrs.org


 February 16, 2026
February 16, 2026 









