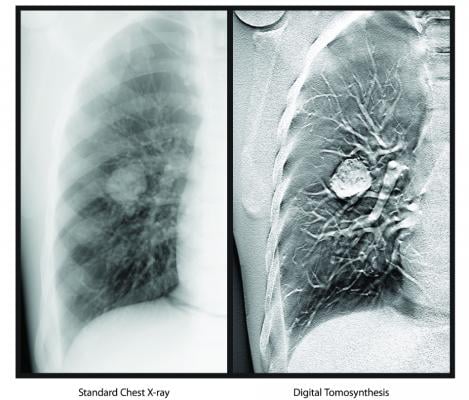
January 15, 2020 — Carestream’s X-ray digital tomosynthesis (DT) functionality, which creates three-dimensional datasets from digital radiography (DR) that can be scrolled through similar to computed tomography (CT) imaging, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
Simplifying workflow and reducing exam time, DT technology is an upgradable option on the Carestream DRX-Evolution Plus System, a versatile digital radiography system that can perform a wide range of general radiographic exams.
Digital tomosynthesis uses a single sweep of X-ray exposures and streamlines operator workflow by separating the process of DT exposure acquisition from image volume formation. As a result, it can generate data from a series of low dose X-ray images of the same organ, taken at the same X-ray exposure, from different angles.
Similar to a CT scan, DT can produce cross-sectional images of an organ, allowing for increased visibility. The radiologist can scroll through the stacked images in the dataset to better define what is being seen and the exact location of areas of interest. This capability enhances the DRX-Evolution Plus in situations where physicians need quick answers.
In trauma centers, CT rooms might be overbooked and there might be a wait, but this technology allows further examine any body part, including the chest or lungs. Digital tomosynthesis generates many image slices, which helps sharpen diagnosis and make medical treatment more efficient. Carestream said digital tomosynthesis does not replace CT, but it can be used in conjunction with it.
Carestream customers have the option to purchase the DT software for their DRX-Evolution Plus systems.
See an example of this technology in the VIDEO: Editors Choice of the Most Innovative New Radiology Technology at RSNA 2019. The Carestream segment starts at 5:10.
FDA Clears New Dual-energy X-ray Technology From Carestream - another new feature on the same X-ray system.
For more information: www.carestream.com/en/us/medical


 February 16, 2026
February 16, 2026 









