July 18, 2017 — National Decision Support Company announced the full qualification of its CareSelect Platform as a Qualified Clinical Decision Support Mechanism (qCDSM) by the Centers for Medicare and Medicaid Services (CMS). The qualification indicates compliance with the Appropriate Use Criteria (AUC) provisions under the Protecting Access to Medicare Act (PAMA) and the Medicare Access and CHIP Reauthorization Act (MACRA).
On June 20, 2017, CMS released the MACRA proposed rule and specified that Merit-based Incentive Payment System (MIPS)-eligible clinicians will receive credit in the practice improvement category. When implemented within certified electronic health record technology (CEHRT), clinicians will receive credit towards their Advancing Care Information (ACI) performance score for consultation of the CDSM across all advanced diagnostic imaging services.
On July 13, CMS released the CY2018 Medicare Physician Fee Schedule (MPFS) proposed rule, including an updated list of Provider Led Entities and a list of qualified CDSMs. CDSMs can be approved for two levels of qualification — preliminary or full. Preliminary qualification indicates that the mechanism does not yet meet the requirements as outlined by CMS. Full qualification indicates that the mechanism does meet the requirements as outlined by CMS and can be used for compliance with PAMA and MACRA. NDSC's CareSelect qCDSM meets all the requirements as outlined by CMS for full qualification.
In the CY2018 MPFS Proposed Rule, CMS reiterated and clarified that consultation of a qCDSM is required for all applicable advanced diagnostic imaging exams and provided important information about the required consultation data for claims to receive payment.
"What's still missing from this program is how CMS intends to flag ordering providers as outliers. Providers need to be aware that both the adherence to the Appropriate Use Criteria (AUC), as well as the applicability of the AUC to their scope of practice are both opportunities for CMS to flag providers as outliers," said Robert Cooke, vice president of marketing at NDSC. "A comprehensive consultation requirement translates to comprehensive measurement. qCDSMs must report not only on adherence to AUC, but also applicability of the AUC to their scope of practice. If an organization implements a CDSM that does not deliver AUC that cover the providers scope of practice, there is a risk that many consultations will not yield applicable AUC, and thus increase the likelihood that these providers will be subject to the additional burden of prior-authorization."
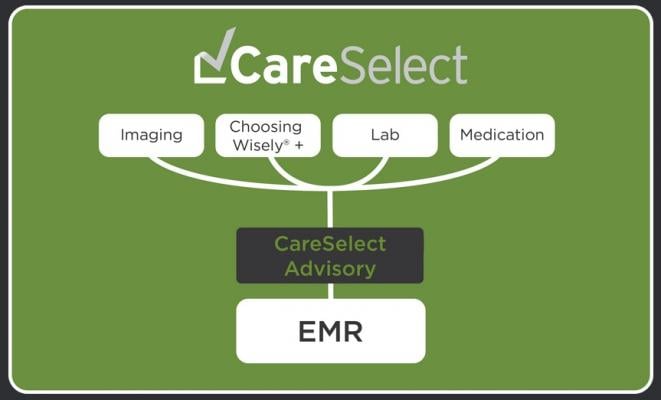
CareSelect Imaging delivers a comprehensive and growing set of structured indications and AUC from five qPLEs including the American College of Radiology (ACR Select), the American College of Cardiology (ACC), the National Comprehensive Cancer Network (NCCN) and the Society of Nuclear Medicine and Molecular Imaging (SNMMI). In addition, NDSC is collaborating with healthcare organizations that are qualified by CMS as qPLEs to utilize NDSC's CareSelect Platform to deliver their AUC. These organizations will author their own AUC for delivery through the CareSelect CDSM.
These clinical guidelines are rationalized into NDSC's CareSelect Platform to enable full compliance with the AUC provisions of PAMA and MACRA. NDSC's comprehensive clinical coverage ensures that providers can always find the correct clinical scenario for every advanced imaging exam including all Priority Clinical Areas (PCAs). This ensures that a payable claim is generated for every advanced imaging exam.
"The reporting requirements outlined in rulemaking are a subset of the data that the qCDSM must record. Combined with the requirement that CDSM must store data for 6 years, we expect that the data recorded in the mechanism will be subject to audit," said Cooke. "Qualification indicates that the technology meets some minimum standard, including AUC coverage. This minimum standard does not consider the scope of practice for a provider. NDSC is engaged with over 500 health systems representing over 2,500 discrete facilities, delivering tight integrations with almost all major EMR platforms. When considering a strategy for compliance, ensuring that AUC covering the full scope of practice is a critical decision point for caregivers when implementing a qCDSM."
For more information: www.nationaldecisionsupport.com


 February 11, 2026
February 11, 2026