Greg Freiherr has reported on developments in radiology since 1983. He runs the consulting service, The Freiherr Group.
RSNA 2017: Often Real Systems (Were) Not Available
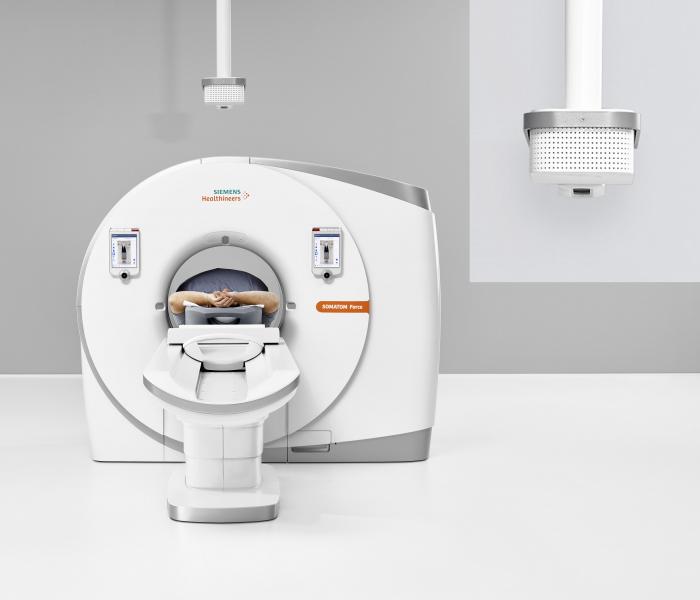
Hanging from the ceiling above a premium CT scanner, Siemens’ FAST (Fully Assisting Scanner Technologies) 3D Camera is designed to optimize patient positioning using artificial intelligence to evaluate optical and infrared pictures of the patient on the CT table below. Shown at RSNA 2017, the work in progress had not yet been cleared by the FDA for marketing in the U.S.
In the run-up to RSNA 1999, General Electric unveiled its 0.7T OpenSpeed. It was easy to be impressed.
The conference was held amid the haunts of Saturday Night Live and NBC Network News. (At the time, GE owned NBC, as well as GE Medical Systems.) It was hosted by GE Medical President and CEO Jeffrey Immelt (who would later become president and chairman of GE). And it featured the world’s first “high-field” open MR scanner.
What the other writers and I saw was not the real thing but an OpenSpeed mock-up. Three weeks later we saw it again (or one very similar to it), rotating on a stage in the center of the 2000-square-foot second floor (yes, “second” floor) of GE Medical’s booth at RSNA 1999.
GE was not the only exhibitor that knew how to put on a show. Vendors had become so skilled at smoke and mirrors that the abbreviation “RSNA” had come to mean (jokingly) “Real Systems Not Available.”
The joke had been around for more than a decade (see “Real Systems Not Available: Punch Line or Prophecy?” , Imaging Technology News, Nov. 1, 2016). But after the launch of OpenSpeed, it reached new heights of comedy.
Siemens Medical Systems (today Siemens Healthineers) had interpreted GE’s OpenSpeed news conference as an attempt to win over the radiology community with its prowess in MR. Not to be outdone, Siemens brought its own mock-up to the exhibit floor of RSNA 1999, one that promised a field strength beyond that of the OpenSpeed (1T versus 0.7T). But there was a problem. When I went to lean up against it, a member of the company’s PR staff stopped me. The mock-up, she said, was made of balsa wood.
Siemens had been talking about a 1T open MR scanner for months. But GE’s “unveiling” of OpenSpeed lit a fire under the company to do more. In the three weeks between GE’s news conference and the opening of the RSNA exhibit floor, Siemens glued together a balsa mock-up of how the 1T was expected to look.
In retrospect, imaging’s version of the Spruce Goose might have been a bit over the top. But mockups were what companies did back then. Competition was fierce. And the race to develop high-field open scanners was especially so.
Just a few years earlier, the MR market was in free fall. Open MR sales had turned it around. Vendors did so with only low- and midfield units. High-field open MR was the next logical step. The companies working on it didn’t publicly address the technical hurdles. And we in the press were so shell shocked by the technology paraded before us each year that we didn’t question what was possible. MRI was itself astounding, as was multislice CT. And digital X-ray. And PET. And PACS. To put it simply, radiology was magical.
A lot has changed since then. But the RSNA joke persists.
WIPs Today
At RSNA 2017, “works in progress” (WIPs) dotted the exhibit halls. Some were lightning rods for attention. Getting a lot was Siemens’ GOKnee3D software, which promised to cut MR knee exams (usually 20 minutes) in about half. Other Siemens WIPs generated buzz, namely the company’s latest CT scanners — Somatom go. All and go.Top — as well as Siemens’ FAST 3D Camera, a camera-software combination designed to leverage artificial intelligence algorithms to position patients on Siemens’ ultrapremium CTs.
And Siemens was not the only one exhibiting works in progress. Other big-name WIP presenters (and their to-be products) included Philips Healthcare (Prodiva 1.5T) and Canon Group’s Toshiba (Aquilion Precision).
The status of many WIPs typically is due to FDA rules against vendors marketing technologies before clearing the agency’s 510(k) process. Devices going through this process are “substantially equivalent” to ones already on the market. (It never ceases to amaze me how many “breakthroughs” on the RSNA exhibit floor receive 510(k) clearances.)
Reviews should be done 90 days after companies submit applications. But the timelines can extend much longer. For example, reviewers might formally ask vendors questions about devices being reviewed. This can throw a monkey wrench into productization timelines.
This is why vendors don’t usually predict when products “pending FDA clearance” will begin shipping. As one vendor emailed me (with an emoji wink and smile), the FDA “does not like it when companies predict such things.”
But U.S. regulators are only one source of delays. GE’s new premium CT, the Revolution Frontier, has cleared the FDA. Yet, the company doesn’t plan to begin shipping the product until second quarter 2018.
Sometimes delays can be anticipated; sometimes not. Uncertainty has been around for a while.
A Long History Of RSNAs
More than a year after its news conference, OpenSpeed had been installed in fewer than two dozen locations (“Patients Embrace New Generation of Imaging Machines,” New York Times, May 8, 2001). Back then, I had anticipated neither the issues that kept OpenSpeed from being a breakout success nor the difficulty that Siemens would encounter developing its 1T open MR.
Today, all but one vendor has stepped away from the high-field open marketplace in favor of wide-bore scanners. But, when reporting the introduction of OpenSpeed, I wrote – incorrectly – that within the next five to 10 years “open MRI scanners will be available up to, if not beyond, 1.5 Tesla” (“GE brings high-field MRI to open scanning environments,” Diagnostic Imaging, November 1999). Thankfully, I attributed that statement to “industry visionaries.” (Stupid visionaries…)
As the most recent meeting demonstrated, works in progress continue to appear on the RSNA exhibit floor. And they will likely continue to do so for years to come.
So, when you look back at RSNA 2017 and forward to upcoming ones, remember the joke…and smile. I do.


 January 22, 2026
January 22, 2026