October 5, 2012 — Judge Victoria Kaufman of the U.S. Bankruptcy Court for the Central District of California (San Fernando Valley Division) approved Gamma Medica Inc.’s $1.5 million debtor-in-possession (DIP) financing on a final basis on Oct. 3 as part of its Chapter 11 restructuring. This DIP financing order provides the molecular breast imaging (MBI) manufacturer with sufficient financing to pursue a reorganization or asset sale.
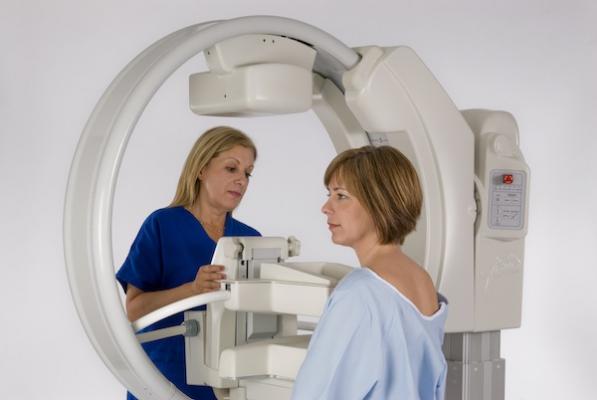
“Gamma Medica’s LumaGEM molecular breast imaging system is already in the market and is very promising,” said recently named CEO Jim Calandra. “We also have industry leading preclinical imaging products. We plan on being sure our tools are in the hands of researchers and radiologists working in women’s imaging."
Gamma Medica’s Clinical Imaging Division, with its LumaGEM MBI system, has installed more than 20 systems in clinical and research facilities in the United States and Europe. Radiologists use the device as a diagnostic tool, adjunct to a traditional mammogram, and it is useful thanks to the ability to diagnose lesions in dense breast tissue. Gamma Medica also has a Pre-Clinical Imaging Division with its Triumph II tri-modality imaging system, which features the only solid-state SPECT (single-photon emission computed tomography) imaging available and market-leading PET (positron emission tomography) imaging technology, available for small animal imaging.
Gamma Medica has also hired investment bank CRS Capstone LLC to advise the company on the sale or financial restructuring of one or more of Gamma Medica’s divisions. “We were already restructuring Gamma Medica when the company filed for Chapter 11 protection, so we are already on the path to emergence,” said Calandra, founder of the restructuring firm Birch Hill Partners. “We’ve made changes in management and we’re already refocusing operations on the needs of our customers.”
“It is unusual for a startup company to have the creditor support to be able to go through Chapter 11 rather than liquidation, but the LumaGEM technology has been embraced in the market and its investors expect it to succeed,” added Calandra. “Its reimbursement is half the cost of MRI [magnetic resonance imaging] and radiologists tell us the images are much easier and faster to read.”
For more information: www.gammamedica.com


 February 20, 2026
February 20, 2026 









