September 22, 2017 — Augmenix Inc. announced a preview of clinical data that will be presented at the 59th Annual Meeting of the American Society for Radiation Oncology (ASTRO 2017), Sept. 24-27 in San Diego. The presentations will provide new insights on the clinical and quality of life benefits of the company’s novel absorbable hydrogel spacing technology in providing protection during radiation therapy.
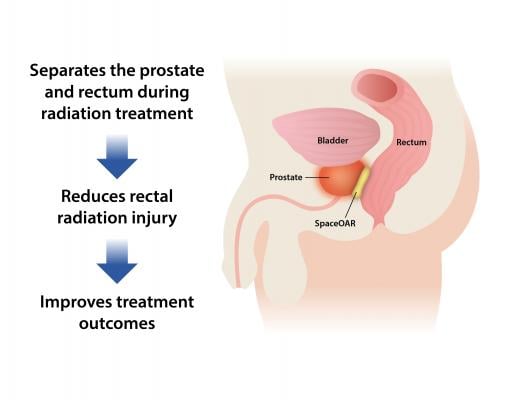
SpaceOAR hydrogel, Augmenix’s lead product, is U.S. Food and Drug Administration (FDA)-cleared and is designed to protect the rectum in men undergoing radiation therapy for prostate cancer.
Most notably at ASTRO 2017, results from the first prospective study evaluating the efficacy of SpaceOAR hydrogel in patients undergoing high-dose stereotactic body radiation therapy (SBRT) for prostate cancer will be presented as a late-breaking clinical trial on Tuesday, Sept. 26. Additionally, the first five-year quality of life data results in prostate cancer patients treated with SpaceOAR hydrogel will unveiled the same day in an oral presentation, demonstrating significant bowel and sexual function benefits. A didactic industry-sponsored panel discussion on the present and future role of hydrogel spacing in the treatment of prostate cancer, led by renowned radiation oncologists from around the world, will occur on Monday, Sept. 25.
Following is a detailed schedule of the company’s events in chronological order (times are U.S. Pacific Daylight Time):
Sunday, September 24
The following poster presentations will take place from 1:15 p.m. – 2:45 p.m. in the Poster Hall:
- Poster Presentation (No : 2559): Dosimetry for Organs at Risk With and Without Use of Perirectal Hydrogel Spacer in Prostate Cancer Patients Treated With SBRT
- Presented by: Daniel B. Fried, M.D., Ph.D., MPH
- Poster Presentation (No : 2572): A Pilot Study – The Role of Radio-Opaque Hydrogel Tissue Marker in the Treatment of Postprostatectomy Intensity Modulated Radiation Therapy
- Presented by Huong Ho, DO
- Poster Presentation (No : 3383): Updated Retrospective Dose Volume Histogram Analysis of High Dose Rate Prostate Brachytherapy Patients With Hydrogel Spacer Implantation
- Presented by Sean Cavanaugh, M.D.
Monday, September 25
- Industry-Expert Theatre: Present and Future Role of Hydrogel Spacing in the Treatment of Prostate Cancer – 3 & 5 Year QOL Evidence and Stereotactic Body Radiation Therapy (SBRT) Experience
- Time/Location: 12:30 p.m. – 1:30 p.m. Exhibit Hall – Theatre 1
- Speakers: Robert Timmerman, M.D., professor of radiation oncology and neurology at the University of Texas Southwestern Medical Center in Dallas; Michael Zelefsky, M.D., professor of radiation oncology, vice-chair of the Department of Radiology and chief of the Brachytherapy Service at Memorial Sloan-Kettering Cancer Center in New York City; Daniel Hamstra, M.D., Ph.D., clinical director and professor at William Beaumont Oakland University Medical School, Department of Radiation Oncology, Beaumont Health, Dearborn, Mich.; and Prof. Dr. Michael Pinkawa, director of the Department of Radiation Oncology, MediClin Robert Janker Klinik in Bonn, Germany.
- Moderator: Rodney Ellis, M.D., vice chair for clinical affairs at the University Hospitals Cleveland Medical Center and associate professor, radiation oncology at Case Western Reserve University in Cleveland.
- Poster Presentation (No : 3613): The Rectal Dosimetric Effects of Perirectal Hydrogel Spacers in Men Undergoing Prostate Stereotactic Body Radiation Therapy (SBRT)
- Time/Location: 4:15 p.m. – 5:45 p.m. Poster Hall
- Presenter: Shaan Kataria, M.D.
Tuesday, September 26
- Late-Breaking Abstracts Special Session (SS 22): Multi-Institutional Phase 2 Trial of High-Dose Stereotactic Body Radiation Therapy with Temporary Hydrogel Spacer for Low- and Intermediate-Risk Prostate Cancer
- Time/Location: 7:45 a.m. – 9 a.m. Room 5A
- Presenter: Michael Folkert, M.D.
- ePoster Presentation (No: 1083): Quality of Life after Radiation Therapy for Prostate Cancer With a Hydrogel Spacer: Five Year Results
- Time/Location: 1:12 p.m. Room 5B
- Presenter: Michael Pinkawa, M.D., Ph.D.
- Poster Presentation (No : 2869): Biodegradable Hydrogel Spacer Injection for Contralateral Submandibular Gland Sparing in Radiation Therapy for Head and Neck Cancers
- Time/Location: 2:45 p.m. – 4:15 p.m. Poster Hall
- Presenter: Avani Rao, M.D.
- Poster Presentation (No : 2435): Novel Use of a Hydrogel Spacer to Separate the Head of the Pancreas and Duodenum for Radiation Therapy for Pancreatic Cancer
- Time/Location: 4:45 p.m. – 6:15 p.m. Poster Hall
- Presenter: Avani Rao, M.D.
Read "Augmenix Announces Positive Three-Year Long-Term Data for SpaceOAR Hydrogel Spacer"
For more information: www.augmenix.com


 February 04, 2026
February 04, 2026 









