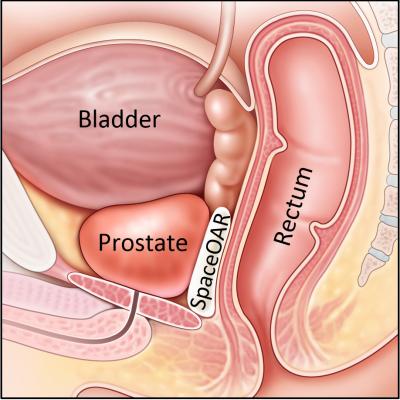
January 16, 2017 — Augmenix Inc. announced that the International Journal of Radiation Oncology Biology and Physics (IJROBP), also known as the Red Journal, has published long-term outcomes data from the company’s Phase 3 clinical trial for SpaceOAR System. SpaceOAR is the first absorbable hydrogel spacer designed to separate the rectum and prostate during prostate cancer radiotherapy.
The Red Journal publication reports three-year post-treatment data in the Phase 3 prospective, randomized, multi-center, patient-blinded clinical trial of the SpaceOAR System. The study evaluated radiation dose to critical structures (prostate, rectum, bladder, penile bulb), rectal and urinary toxicity, and quality of life (QOL) impact on prostate cancer patients treated with and without SpaceOAR System during radiotherapy. For QOL, the Expanded Prostate Cancer Index Composite (EPIC) was utilized. The SpaceOAR System is the only product that is U.S. Food and Drug Administration (FDA)-cleared to provide a barrier between the prostate and rectum to decrease toxicity and minimize changes in QOL following prostate radiotherapy. It is injected as a liquid and then solidifies into a soft hydrogel that pushes the rectum out of the high-dose prostate radiation field for three months during radiation therapy. The hydrogel is absorbed by the body in the months following treatment.
During radiotherapy the spacer resulted in a 73.5 percent reduction in rectal V70 radiation dose, and a 49 percent reduction in median penile bulb radiation dose in patients treated with SpaceOAR (SpaceOAR) compared to men who did not receive SpaceOAR hydrogel (Control). In the three years following radiation treatment the SpaceOAR group demonstrated a 78 percent reduction in late rectal toxicity complications, compared to Control patients. No patients in the SpaceOAR group (0 percent) experienced grade two or worse rectal toxicity, compared to 5.7 percent in the Control group. Results also showed a 75 percent reduced risk of mild urinary incontinence among patients treated with SpaceOAR compared to Control. At three years, the average SpaceOAR patient bowel and urinary QOL measure was the same as before radiotherapy, while the Control patients QOL measures had significantly declined.1
Overall patient wellness at three years was also assessed by looking at the percent of patients with clinically significant declines in all three QOL areas (bowel, urinary and sexual). Fully 20 percent (one in five) of the Control patients had clinically significant QOL declines in all three QOL areas, compared to only 2.5 percent (one in forty) of the SpaceOAR patients.
“These results further validate the safety and efficacy of the SpaceOAR System and highlight the long-term benefits it can provide to prostate cancer patients who are treated with radiotherapy,” said Daniel Hamstra, M.D., Ph.D., radiation oncologist at Texas Center for Proton Therapy. “By creating a space between the rectum and prostate, SpaceOAR can help prevent risks of injury to surrounding healthy tissue during radiation, representing an important advantage for thousands of men treated for prostate cancer each year.”
For more information: www.spaceoar.com
References


 January 30, 2026
January 30, 2026 









