November 10, 2016 — Augmenix Inc. announced that the American Medical Association (AMA) has granted a Category I, Current Procedural Terminology (CPT) code specifically for periprostatic implantation of biodegradable material. AMA granted the code with the support of the American Society for Radiation Oncology (ASTRO) and the American Urological Association (AUA).
The purpose of CPT is to provide a uniform language that accurately describes medical, surgical and diagnostic services, and thereby serves as an effective means for reliable nationwide communication among physicians and other healthcare providers, patients and third parties. The new CPT code is expected to become effective on Jan. 1, 2018 at which time the Category III code, 0438T, will be eliminated.
Following the recent presentation of positive three-year data from the SpaceOAR Pivotal Clinical Trial at the 2016 ASTRO Annual Meeting in Boston, AMA granted a Category 1 CPT code for periprostatic implantation of biodegradable material.
“SpaceOAR is clearly the most elegant and clinically proven technology for reducing rectal toxicity in the treatment of prostate cancer developed in the past 20 years,” said Steven Kurtzman, M.D., president, Western Radiation Oncology Inc., San Mateo, Calif. “I consider the product an integral part of my treatment protocol for patients receiving both external beam radiation therapy and brachytherapy.”
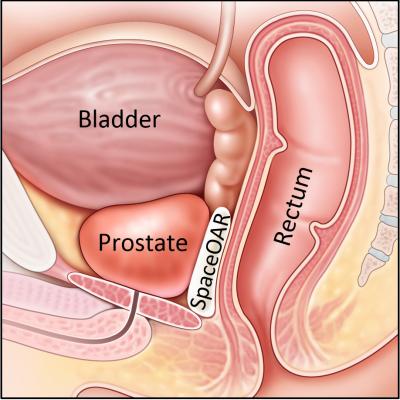
The leading side effects of prostate cancer radiotherapy are collectively known as “rectal toxicity” (diarrhea, rectal bleeding, urgency, pain, etc.), which results from unintended radiation injury to the rectum (the Organ At Risk, OAR). These complications can last for years, significantly impacting patient quality of life (QOL).
Using a minimally invasive procedure, Augmenix’s SpaceOAR System is injected as a liquid into the space between the prostate and rectum where it expands the space and then solidifies into a soft hydrogel. The hydrogel remains stable for three months while protecting the rectum during radiotherapy, and then liquefies and is completely absorbed by the body after radiotherapy is complete.
The SpaceOAR System is U.S. Food and Drug Administration (FDA)-cleared. It is also CE marked, approved in Australia and licensed in Canada.
For more information: www.augmenix.com


 February 17, 2026
February 17, 2026