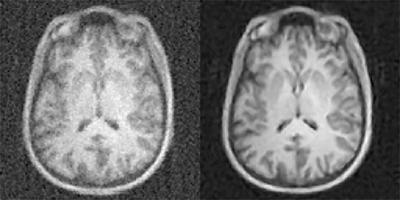
A new artificial-intelligence-based approach to image reconstruction, called AUTOMAP, yields higher quality images from less data, reducing radiation doses for CT and PET and shortening scan times for MRI. Shown here are MR images reconstructed from the same data with conventional approaches, at left, and AUTOMAP, at right. Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital
July 17, 2018 — A research team with funding from the National Institute for Biomedical Imaging and Bioengineering (NIBIB) has developed an advanced computing technique for rapidly and cost effectively improving the quality of biomedical imaging. The technology, called AUTOMAP, uses machine learning and software, referred to as neural networks — inspired by the brain’s ability to process information and perceive or make choices. AUTOMAP finds the best computational strategies to produce clear, accurate images for various types of medical scans.
In their study in the March 21, 2018, issue of Nature, the researchers from Massachusetts General Hospital (MGH) Martinos Center for Biomedical Imaging and Harvard University found that the AUTOMAP system could produce brain magnetic resonance imaging (MRI) images with better signal and less noise than conventional MRI techniques. Achieving a good signal-to-noise ratio is a key factor in generating a quality MRI scan.
MRI uses a magnetic field and radio waves to create detailed images of tissues inside the body. Noise from either electronic sources or from tissues in the body are detrimental to image quality, so imagers look for ways to lessen its effect.
“The signal-to-noise ratio improvements we gain from this artificial intelligence-based method directly accelerates image acquisition on low-field MRI,” said lead author Bo Zhu, Ph.D., postdoctoral research fellow in radiology at Harvard Medical School and in physics at the MGH Martinos Center. NIBIB has supported Zhu’s postdoctoral research on this project. He added that the AUTOMAP neural network will be compatible with novel image acquisition strategies and unconventional hardware designs.
AUTOMAP churns through — and learns from — data from existing images and applies mathematical approaches in reconstructing new ones. The team used a set of 50,000 MRI brain scans from the NIH-supported Human Connectome Project to train the AUTOMAP system to reconstruct images in their study, successfully demonstrating improvements in reducing noise and reconstruction artifacts over existing methods.
AUTOMAP achieves almost instantaneous image reconstruction, according to senior author Matt Rosen, Ph.D., director of the Low-field MRI and Hyperpolarized Media Laboratory and co-director of the Center for Machine Learning at the MGH Martinos Center. The reason for the rapid processing speed — just tens of milliseconds — is that the neural network has no cycles or loops, rather is a feedforward system.
“Some types of scans currently require time-consuming computational processing to reconstruct the images,” Rosen said. “In those cases, immediate feedback is not available during initial imaging, and a repeat study may be required to better identify a suspected abnormality. AUTOMAP would provide instant image reconstruction to inform the decision-making process during scanning and could prevent the need for additional patient visits.”
“This technology could become a game changer, as mainstream approaches to improving the signal-to-noise ratio rely heavily on expensive MRI hardware or on prolonged scan times,” said Shumin Wang, Ph.D., director of the NIBIB program in magnetic resonance imaging. “It may also be advantageous for other significant MRI applications that have been plagued by low signal-to-noise ratio for decades, such as multi-nuclear spectroscopy.”
For more information: www.nature.com/nature
Reference
Zhu B., Liu J.Z., Cauley S.F., et al. "Image reconstruction by domain-transform manifold learning." Nature, March 21, 2018. https://doi.org/10.1038/nature25988


 February 13, 2026
February 13, 2026 









