November 15, 2017 — Arterys Inc. announced the close of its $30M Series B financing round. The investment was led by Temasek, with strategic investors Northwell Health Ventures, NewYork-Presbyterian, Varian Medical Systems and GE Ventures, and joined by Fosun, DNA Capital, Emergent Medical Partners and ORI Capital. Arterys plans to use the funding to expand its web-based artificial intelligence (AI) platform, and launch several products in oncology and neurology, and to accelerate the commercialization of its cardiac imaging offering. This new round will enable more radiologists to benefit from artificial intelligence in their daily work.
Arterys has created a novel AI platform for medical image analytics. Contrary to the current solutions that are installed inside hospitals, Arterys software is web-based. As a result, physicians benefit from vast amounts of computation, which is necessary to run artificial intelligence algorithms in a timely manner. Physicians can now process their patient cases with support from AI algorithms that are embedded in their workflow. This is possible thanks to proprietary technology that protects patient information and complies with data privacy regulations in the United States and European Union.
"We were impressed with the Arterys approach, fusing the latest cloud and artificial intelligence technologies with a true user-centric design methodology. This results in augmented workflows with increased efficiency and consistency, while the radiologist remains in charge of the final clinical decision," said Thomas Thornton, senior vice president of Northwell Health Ventures.
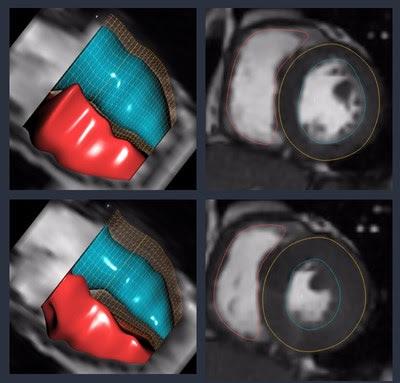
The Arterys cloud platform supports a variety of imaging modalities, such as magnetic resonance imaging (MRI) and computed tomography (CT). It provides a solid foundation for seamless image interpretation with artificial intelligence tools, case sharing and automated reporting. The platform is designed to capture user edits on the results of the machine learning, so that algorithms can be improved based on real-world use. Working together, these capabilities enable the company to quickly build and commercialize new product offerings.
Arterys started with cardiac MRI, one of the most complex and time consuming workflows, to demonstrate the power of a combined artificial intelligence and cloud computing approach. It was the first product its kind to obtain U.S. Food and Drug Administration (FDA) clearance for use as a diagnostic support tool, and is also cleared in Canada and the EU. The software has significant benefits for patients and clinicians, reducing the time the patient needs to be in the scanner, and automating key clinical measurements for the radiologist.
For more information: www.arterys.com


 February 20, 2026
February 20, 2026 









