Jan. 15, 2013 -- Eli Lilly and Company and Avid Radiopharmaceuticals, Inc., a wholly owned subsidiary of Lilly, announced today that Amyvid (Florbetapir F 18 Injection) has received marketing authorization from the European Commission as a diagnostic radiopharmaceutical indicated for Positron Emission Tomography (PET) imaging of beta-amyloid neuritic plaque density in the brains of adult patients with cognitive impairment who are being evaluated for Alzheimer's disease and other causes of cognitive impairment. Amyvid should be used in conjunction with a clinical evaluation.[1]
Alzheimer's Disease is one of many possible causes of cognitive impairment, which can make diagnosis challenging.[2],[3] Alzheimer's Disease and other causes of cognitive impairment share many overlapping symptoms, including deficiencies in memory, visuospatial ability, executive function, behavior and language.[2],[3] It is estimated that up to one in five patients clinically diagnosed with probable Alzheimer's Disease during life do not exhibit Alzheimer's Disease pathology upon autopsy.[4],[5]
"We believe that Amyvid fills an unmet need in the medical community, providing physicians with important information about the presence or absence of beta-amyloid plaques that can help identify the cause of their patients' cognitive symptoms," said Diane Bakaysa, Amyvid global brand development leader. "This is important because, if, based on negative Amyvid findings and clinical assessment, it is determined that Alzheimer's Disease is not the cause of cognitive impairment, a physician can avoid unnecessary or potentially harmful treatments associated with a misdiagnosis of Alzheimer's Disease."[6],[7],[8]
Beginning in Q2 2013, Amyvid will be available in select areas within the European Union.
Amyvid for intravenous use was approved by the U.S. Food and Drug Administration (FDA) in April 2012 and is supplied in 10 mL, 30 mL or 50 mL multidose vials containing 500–1,900 MBq/mL Florbetapir F 18.[9] Amyvid is indicated for PET imaging of the brain to estimate beta-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer's Disease (AD) and other causes of cognitive decline.[9]
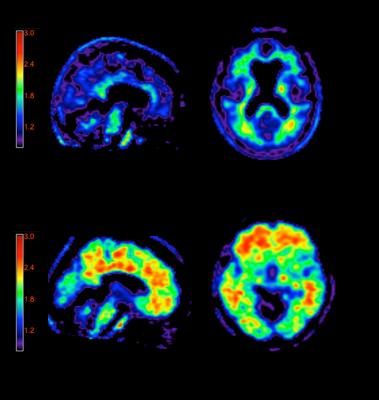
A negative Amyvid scan indicates sparse to no neuritic plaques and is inconsistent with a neuropathological diagnosis of AD at the time of image acquisition; a negative scan result reduces the likelihood that a patient's cognitive impairment is due to AD. A positive Amyvid scan indicates moderate to frequent amyloid neuritic plaques; neuropathological examination has shown this amount of amyloid neuritic plaque is present in patients with AD, but may also be present in patients with other types of neurologic conditions as well as older people with normal cognition.[9]
Amyvid is an adjunct to other diagnostic evaluations. A positive Amyvid scan does not establish a diagnosis of AD or other cognitive disorder. Safety and effectiveness of Amyvid have not been established for predicting development of dementia or other neurologic condition, or monitoring responses to therapies.[9]
About Amyvid
Amyvid is a radioactive diagnostic agent that is injected into the bloodstream, where it crosses the blood-brain barrier and selectively binds to amyloid plaques. The fluorine 18 (F 18) isotope produces a positron signal, which is detected by a PET scanner.[9],[10] Physicians who read Amyvid PET scans should complete a comprehensive training program available through live events or online at AmyvidTraining.com.[9]
Indications and Usage[9]
In the United States, Amyvid is indicated for positron emission tomography (PET) imaging of the brain to estimate beta-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer's Disease (AD) and other causes of cognitive decline.
A negative Amyvid scan indicates sparse to no neuritic plaques and is inconsistent with a neuropathological diagnosis of AD at the time of image acquisition; a negative scan result reduces the likelihood that a patient's cognitive impairment is due to AD. A positive Amyvid scan indicates moderate to frequent amyloid neuritic plaques; neuropathological examination has shown this amount of amyloid neuritic plaque is present in patients with AD, but may also be present in patients with other types of neurologic conditions as well as older people with normal cognition. Amyvid is an adjunct to other diagnostic evaluations.
Limitations of Use:
A positive Amyvid scan does not establish a diagnosis of AD or other cognitive disorder. Additionally, the safety and effectiveness of Amyvid have not been established for predicting development of dementia or other neurologic condition, or monitoring responses to therapies.
[1]Amyvid [package insert]. European Union. Eli Lilly & Co.; 2013.
[2]Balasa M, Gelpi E, Antonell A, et al; for the Neurological Tissue Bank/University of Barcelona/Hospital Cl nic NTB/UB/HC Collaborative Group. Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology. 2011;76(20):1720–1725.
[3]Alzheimer's Association. 2012 Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8(2):131–168.
[4] Lim A, Tsuang D, Kukull W, et al. Clinico-neuropathological correlation of Alzheimer's disease in a community based case series. J Am Geriatr Soc. 1999;47(5):564–569.
[5] Petrovitch H, White LR, Ross GW, et al. Accuracy of clinical criteria for AD in the Honolulu-Asia Aging Study, a population-based study. Neurology. 2001;57(2):226–234.
[6]Alzheimer's Association. 2011 Alzheimer's disease facts and figures. Alzheimers Dement. 2011;7(2):208–244.
[7] Boise L, Neal MB, Kaye J. Dementia assessment in primary care: results from a study in three managed care systems. J Gerontol A Biol Sci Med Sci. 2004;59(6):621–626.
[8] Mendez MF, Shapira JS, McMurtray A, et al. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007;15(1):84–87.


 February 13, 2026
February 13, 2026 









