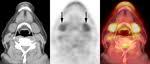
November 5, 2009 - ACRIN seeks sites for the clinical trial: “A Multicenter Trial of FDG-PET/CT Staging of Head and Neck Cancer and its Impact on the N0 Neck Surgical Treatment in Head and Neck Cancer Patient” (ACRIN 6685).
The trial aims are to determine the predictive value of PET/CT for staging the clinically-defined negative neck (N0 neck) based upon pathological sampling of the neck lymph nodes and to determine the potential of PET/CT to change treatment of the N0 neck patient. The study will be led by Val Lowe, M.D., professor of radiology, Division of Nuclear Medicine, at Mayo Clinic, Rochester, Minn.,
Trial activation is anticipated in January 2010 with accrual of the 292 study participants expected in well under two years. As such, interested investigators are encouraged to act quickly to complete the activation requirements to ensure full inclusion in the study.
For the application and activation requirements, per case reimbursement, imaging parameters: www.acrin.org/6685_protocol.aspx.


 October 30, 2025
October 30, 2025 









