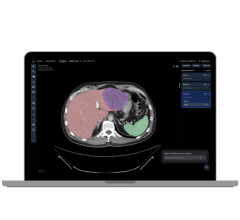
An MRI-guided prostate biopsy showed the presence of cancer for this patient a previous TRUS biopsy was negative.
At 59, George LaVie (not his real name) faced an uncertain future. Based on rising PSA levels (prostate-specific antigen), he had undergone a transrectal ultrasound biopsy (TRUS) to attempt to determine the presence of disease. The results were negative.
George’s story is not unusual. Rising PSA levels are a potential indicator of prostate disease. The standard course of action is a TRUS biopsy. Ultrasound guides the urologist, who takes up to 12 core samples from the gland. In George’s case, the results gave no indication of cancer, so he began “watchful waiting.”
“I went another year or so and my PSA levels continued to rise,” says George. “My urologist recommended that I get another biopsy. I was apprehensive about doing that. ”
Prompted by George’s rising PSA levels and previous negative TRUS biopsy, an alternative imaging modality was considered. A plan was devised to use new MRI-guided prostate biopsy technology to get better anatomical definition and possibly reach a more conclusive diagnosis. One of the key selling points for George was that, with a better indication of the suspect areas, fewer biopsy samples may be required.
George agreed to try the procedure and was sent to Desert Medical Imaging of Indian Wells, Calif., for a diagnostic MRI. An initial MRI indicated there was indeed something in the prostate that didn’t look right, some sort of mass.
George’s urologist, working side by side with Dr. John Feller of Desert Medical Imaging, performed an MRI-guided prostate biopsy. Five samples were taken during the 30-minute procedure, and subsequent pathology results conclusively showed the presence of cancer.
“I chose to have a radical prostatectomy,” says George. “They removed the prostate and felt that there was no surrounding tissue that was affected by the cancer, so they gave me a good prognosis.”
He continues, “Quite frankly, if this new procedure hadn’t been available, I may have chosen to wait and see and not even have had the biopsy done. And if I had waited, there’s no telling where the cancer would have spread.”
Case study supplied by Invivo Corp.


 February 11, 2026
February 11, 2026