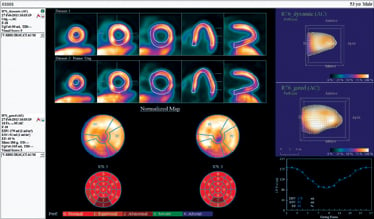
Dynamic scanning images illustrate potential image quality of fatty acid uptake using PET/CT with FluoroPharma's CardioPET.
The next generation of positron emission tomography (PET) imaging agents will herald an age when PET will eclipse single photon emission computed tomography (SPECT) as the “go to” modality for molecular imaging. It will do so by enabling personalized medicine through precision diagnostics, the ability to be delivered cost-effectively in a manner with less radiation to patients, by leveraging hardware advances already being commercialized, and by taking advantage of the extra throughput capacity present in the U.S. installed base of PET/CT scanners.
Fluorodeoxyglucose (FDG) created the oncology business for PET, and its growth was explosive. But while FDG is clinically very good, it is not perfect. It does not work on all cancers and only provides a look at the basic metabolism of tissues. This is opening the door for the next generation of
oncology agents that are specific to cancers not well characterized by FDG.
While there is buzz around new PET agents in neurology for detecting beta amyloid plaques associated with Alzheimer’s disease, it is cardiology that presents some of the most exciting opportunities for PET. New cardiac PET agents promise robust, reproducible and dependable results in everyday cardiology practices. The need for these agents is mind-boggling.
Cardiovascular disease is the single largest cause of global mortality, causing more than 17 million deaths each year, exacting an annual financial cost around the globe of a staggering $863 billion. In the United States alone, 729,000 people die from cardiovascular disease annually. Annual U.S. health costs are estimated to be $280 billion, rising to $800 billion by 2030.
PET vs. SPECT Technology
The future success of PET is grounded in its inherently better image resolution. In cardiac scanning, it has generally been reported that PET offers a resolution of 5 – 7 mm, compared with a cardiac SPECT resolution of 12 – 15 mm. This better performance has allowed data to emerge suggesting that as many as one in 10 scans interpreted as normal on SPECT would have been abnormal if done on PET due to the presence of unseen microvascular, triple-vessel disease. PET’s superior diagnostic capability is achieved partly through advances in hardware, particularly quantification, which leverages numerical precision to identify global perfusion defects in the heart that otherwise might be hidden from qualitative SPECT scans.
PET has an ace in the hole in its ability to quantify results. Quantification is now being built into PET/CTs sold into mainstream medical practices. SPECT scanners lack the resolution of PET/CTs and SPECT vendors have yet to commercialize systems capable of quantification.
A big difference between the two technologies is the half-life of the radiopharmaceutical tracers each uses. SPECT tracers have a long half-life (technetium-99m has a half-life of six hours), whereas rubidium-82 is only 75 seconds. This short half-life is a limitation of the current front-line cardiac PET radiotracer, which does not leave much room for error when imaging and presents the inability to do exercise stress testing.
New PET Radiotracers
Rubidium-82 technology has established benchmarks in the assessment of myocardial perfusion against which the next generation of cardiac agents can be measured for sensitivity and specificity. It also is producing data that should demonstrate the cost-effectiveness of cardiac PET over SPECT, and has pioneered the way for cardiac PET through the issuance of CPT codes and reimbursement policy by third-party payers for PET myocardial perfusion. But rubidium-82 has drawbacks that may limit its sustained clinical adoption as seen in recent publications. These limitations include supply chain issues, economics, its short half-life and potential safety issues as presented in the black box warning in the package insert. These issues should be resolved by the coming wave of new fluorinated cardiac PET radiopharmaceuticals.
Fluorinated positron emitters that compose the next generation of PET radiopharmaceuticals offer benefits over rubidium. They also offer some clinical possibilities in the diagnosis and monitoring of cardiovascular disease beyond myocardial perfusion. Expected advantages of these new agents over SPECT radiopharmaceuticals are improved diagnostic accuracy, image clarity and lower radiation exposure to patients.
FluoroPharma’s BFPET, a fluorinated myocardial perfusion agent, exemplifies how the next generation of cardiac PET technology might boost diagnostic performance. In a study patient, BFPET indicated that the patient did not have a perfusion defect, as was suspected on the basis of SPECT, but rather suffered from apical thinning. Cardiovascular disease was confirmed through CT angiography — as was the absence of a perfusion defect.
BFPET has the potential to establish a new standard for measuring cardiovascular blood flow. The agent is being developed for use in combination with stress testing in patients with presumptive chronic coronary artery disease (CAD), as a replacement for SPECT in institutions with PET capability.
As we enter healthcare reform and its emphasis on cost-effective care, it becomes increasingly important to minimize the number of tests performed. The significance of getting it right the first time rises when we consider the danger of cumulative radiation exposure to the patient, where the best diagnostic test done first might eliminate the need for subsequent invasive, expensive or unnecessary tests.
Next generation cardiac PET agents become even more transformational when they are designed to address unmet clinical needs. Whereas BFPET promises increased diagnostic accuracy compared with thallium and technetium-based SPECT agents, as well as advantages that go beyond the PET agent rubidium-82, CardioPET transcends even that. This agent, another in FluoroPharma’s pipeline, accesses a novel metabolic pathway, one that involves fatty acids, the primary source of energy for the cardiac muscle.
Our studies indicate that imaging with CardioPET can potentially be used to accurately gauge fatty acid uptake by the myocardium. This uptake can be visualized and quantified using PET scanners that are becoming more available at price points within the grasp of routine practitioners.
Preliminary data indicate that the agent is especially suited for the diagnosis of acute coronary syndrome and chronic coronary artery disease in patients who cannot undergo stress testing.
By taking advantage of the power of obtaining list-mode data on modern scanners, it appears as if CardioPET may allow measurements of perfusion during the first five minutes of administration while scans done 40 to 60 minutes later may indicate tissue viability. These two clinical capabilities suggest the potential utility of CardioPET as a way to assess patients after they have complained of chest pain in the emergency department.
(Editor's note regarding new SPECT radiotracers: On the SPECT front, the U.S. Food and Drug Administration (FDA) in March 2013 approved a new indication for GE Healthcare’s agent AdreView (Iobenguane I 123 Injection) as the first agent to link nerve function in the heart to a patient’s mortality risk. AdreView is approved for the scintigraphic assessment of myocardial sympathetic innervation (cardiac nerve activity) to assist in the evaluation of patients with New York Heart Association (NYHA) Class II or Class III heart failure and left ventricular ejection fraction (LVEF) ? 35 percent. Increased myocardial sympathetic activity is a prominent feature of heart failure and is often associated with decline in left ventricular function, worsening heart failure symptoms and sudden cardiac death. This increase leads to a depletion of norepinephrine (NE) storage and uptake. AdreView provides a means for assessing the neuronal capacity for uptake and storage of NE. In January, Lantheus began shipments of its new LEU TechneLite generator, which is the first technetium-99m (Tc-99m) generator in the United States that contains molybdenum-99 (Mo-99) produced from at least 95 percent low-enriched uranium (LEU). Through its supply chain diversification strategy, Lantheus plans to eventually eliminate the use of highly enriched uranium (HEU) sourced Mo-99 from its supply chain. This is also in part due to a federal effort to eventually reduce the availability of HEU to aid interventional efforts to stop nuclear weapons proliferation.)
Imaging Vulnerable Plaques
VasoPET, a next-generation positron biotracer, is being designed to address the unmet clinical need of identifying vulnerable plaques. These plaques are likely to rupture and cause a stroke or heart attack. Preclinical tests show that this fluorine-bearing radiopharmaceutical is taken up by inflammatory cells not found in stable plaque.
This agent is still in preclinical development, so it remains a speculative technology. Nonetheless, the prospect is extraordinarily appealing for an agent that might be used in high-risk patients, particularly those being readied for an intervention such as a cardiac catheterization. This technology could help the interventional cardiologist identify blood vessel segments that have vulnerable plaques to avoid them and the possibility of creating emboli in the vessel. In the future, this imaging might be used to preventively identify and treat these lesions. An imaging agent such as VasoPET would also be helpful in the development of therapeutics being tested for their ability to dissolve vulnerable plaques.
Other modalities, including CT and MRI, cannot differentiate inflamed from stable plaques.
The challenge facing all these agents is whether they can deliver clinically significant results in the hands of routine practitioners and not just once in a while. Their results must be consistent and reproducible, rendering diagnostic and prognostic conclusions regardless of practitioner skill.
Serious Issues With Isotope Supply
The case for PET becomes even more formidable when considering the shortcomings of SPECT compared with PET. Technetium, which fuels the majority of SPECT procedures in cardiology, has suffered several disruptions in supply over the past decade. A reactor at Chalk River Laboratories in Ontario, Canada, normally supplies about half of the world’s molybdenum, which serves as a generator of technetium. The vast majority of the technetium generators are used in the United States. Canada plans to permanently shut down the reactor in 2016 due to its age and safety concerns. Thus far, no other North American sources of molybdenum have surfaced to make up for this deficit.
In the past, supply disruptions have seriously impacted the nuclear medicine community. When a radioactive leak sidelined the reactor in 2009, extreme shortages of technetium resulted over the 15 months the reactor was out of service. Because molybdenum has a half-life of 66 hours, shipments from overseas reactors to the United States caused significant logistical headaches.
Some physicians turned to the PET tracer rubidium-82 to fill in until news broke last year that improper daily monitoring of the generator was resulting in strontium breakthroughs that left radioactive material in the patient long after a perfusion exam. While crossing the border between the United States and Canada in July 2011, two patients who had recently undergone rubidium-82 PET scans set off radiation alarms. It was learned that these patients had been inadvertently exposed to large doses of strontium contaminant, the parent isotope used in the generation of rubidium. Rubidium-82 was voluntarily taken off the market pending review by the FDA and did not return to market until nine months later, after the FDA traced the fault to improper handling of the generator, not its manufacture. The incident has had lasting effects, however, on the molecular imaging community. Today, those using rubidium-82 must undergo special training and are subject to daily reporting requirements. (Editor's note: Currently, the U.S. Department of Energy (DOE) is the only domestic supplier of the isotope strontium-82 used in rubidium-82 generators. Positron Corp. is working with the FDA for certification so it can begin its own production of strontium-82, through its subsidiary Manhattan Isotope Technology, to provide a second source for the PET cardiac perfusion isotope.)
Fluorinated positron emitters could be produced with relative ease in the United States, and the methodology for handling the fluorine-18 radioisotope has been vetted by decades of use and millions of procedures involving FDG without mishap. Fluorine-18 has a lower dose profile than SPECT radioisotopes, reducing the overall radiation burden placed on the patient. Dual isotope SPECT scans may expose the patient to between 25 and 30 mSv of radiation. SPECT rest-stress with technetium exposes the patient to between 8 and 10 mSv. In contrast, exposure from fluorine-18 scans range from 4 to 8 mSv.
To the benefit of the molecular imaging community and the future growth of this modality, the widespread use of FDG with its utility in oncology led to a rapid expansion of the installed base of PET/CT scanners and establishment of an extensive and nationwide FDG distribution network. About 1,800 PET/CTs are operating in the United States today. They are served by about 100 commercial FDG production sites.
Many of these PET/CTs are running at only about half capacity. Consequently, early adopters of the next generation of PET radiopharmaceuticals will have ready access to the needed hardware for performing scans, just as the fluorine-18 isotope will be easily available thanks to the widespread network of FDG production sites.
It makes sense when performing medical imaging procedures to go with the modality that delivers the best image quality at the lowest dose, one that renders reproducible results regardless of the skill of the practitioner and is not vulnerable to supply-line disruptions. The case is that much stronger, when this modality gains access to specialized radiopharmaceuticals that promise cost-effective and clinically significant testing that will expand the capability of routine healthcare practitioners in neurology, oncology and cardiology. itn
Thijs Spoor, FluoroPharma chairman, CEO and president, has more than 15 years medical industry experience. He is a former securities analyst at J.P. Morgan and Credit Suisse, covering the medical device and biotechnology industries. Before joining FluoroPharma, he led the nuclear cardiology portfolio and PET new product opportunity portfolio at Amersham, before its acquisition by GE Healthcare.


 February 13, 2026
February 13, 2026 









