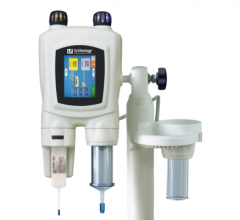
The Bayer/Medrad Mark 7 Arterion angiographic injector is designed for better ergonomics, to be small, light-weigh and easy to use.
Two next-generation automated contrast injector systems were recently introduced in the United States.
In March 2012, Covidien began its global launch of a new version of its Optivantage dual-head computed tomography (CT) contrast media delivery system that allows simultaneous injection. The vendor said simultaneous injection and use of prefilled syringes offer technicians flexibility. The prefilled syringes also help minimize the risk of contamination from filling empty syringes. The system offers Covidien’s Optibolus software to deliver contrast media at a decelerating exponential rate compared to the standard constant rate of uniphasic injections.
At RSNA 2011, Medrad (now Bayer) introduced the Mark 7 Arterion angiographic injection system. The system is much smaller, lighter-weight, more ergonomic and easier to maneuver than previous Medrad injectors. It also is easier to operate than previous models, requiring less time to position and set up. It has a clearly visible and intuitive user interface to guide the user through proper set-up, and highlights the information needed to perform injections.
The Mark 7 Arterion has a unique front loading system to simplify set-up and makes for a cleaner tear down. The Mark 7 Arterion syringe provides a clear view of the contrast.
Bracco Acquires Swiss Medical Care
In January, Bracco Imaging SpA acquired Swiss Medical Care (SMC), a Swiss contrast injector system vendor. The acquisition is a continuation of Bracco’s expansion into the injector market, after previously purchasing Acist and EZEM. The company said Swiss Medical will further strengthen its portfolio of solutions for radiology and diagnostic imaging. Swiss Medical said the merger will help expand sales of its CT Exprés computed tomography syringe-less contrast injector, which gained U.S. Food and Drug Administration approval in December 2006.
Connecting Injector Data to EMRs
At RSNA 2011, Medrad released the Certegra Informatics Connect.RIS vendor-neutral informatics application to establish connectivity between Medrad’s Stellant contrast-injection systems and hospital information systems. The application auto-populates contrast-injection records into reporting systems, eliminating the need to look for or dictate contrast-injection records.


 November 26, 2025
November 26, 2025