Precision medicine is not only a buzzword, it is a call to action to radically advance our capabilities in the era of molecular targeted medicine. Positron emission tomography (PET) has firmly established its leading role as a capable oncologic molecular imaging technique over the last two decades, however its technological advances have been modest at best.
An essential capability of a methodology to support precision medicine with the goal of being tailored to the individual patient and disease characteristics is that such a methodology is a valid biomarker and ideally enables consistent and accurate quantitative readouts. This is an area where PET imaging has been challenged and limited not due to inherent inabilities, but more due to technological limitations.
Technological Challenges
In the days of bragging rights on how many megapixels one’s smartphone has, PET imaging has been lagging behind other cross-sectional imaging methodologies in its ability to improve the precision of the detector chain and the resulting image quality. While computed tomography (CT) imaging is done routinely at cross-sectional image matrixes of 512 x 512, PET imaging has remained nearly unchanged over the last two decades at matrix sizes of 144 x 144 to 200 x 200. This fact gives an insight into some of the technological challenges of conventional PET (cPET) technology.
Another challenge for PET has been the lack of harmonization in acquisition procedures, post-processing and semi- or even quantitative assessment approaches. While considerable progress has been made based on best practices and regulatory and scientific recommendations, variabilities are still largely jeopardizing the robust evolution of PET as a noninvasive, molecular-based technique to enable precision medicine.
Opportunities
Today, radiomics and big data are emerging and highly visible efforts that require precise, high-quality imaging to deliver on this promise of personalized medicine. These big imaging data opportunities are being further nurtured by the availability of decentralized and increasingly cloud-based computational capabilities. Another increasingly important opportunity for PET is its role as a companion diagnostic in the area of tissue markers or genetic profile-driven therapies, as well as its evolving role into integrated theranostic approaches. With such promising and exciting prospects, the introduction and clinical integration of new technologies can drive new PET imaging paradigms.
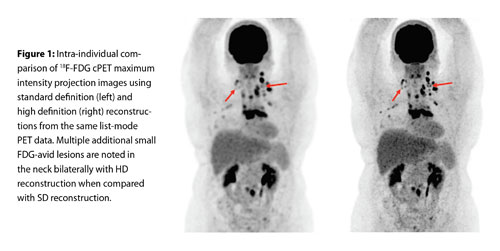
An essential and increasingly accepted PET technology advance has been the refinement of time-of-flight (TOF). This helps to reduce the uncertainty of the true three-dimensional location of the PET isotope annihilation event and thus more precisely localize radiotracer uptake within the body. Current TOF cPET systems can now be improved through the introduction of higher definition reconstructions from the standard definition (SD) PET imaging which uses image matrix sizes of up to 200 x 200, to high definition (HD) of up to 400 x 400, thus reducing the reconstructed cPET image voxel volumes from ~64 mm3 to ~8 mm3. Figure 1 presents a whole-body FDG cPET image using the default SD reconstruction and the new HD reconstruction of the same dataset. The image comparison highlights a visually sharper HD image that has less partial volume effect and more accurate quantification and contributes to improved overall imaging precision.
The backbone of cPET has been photomultiplier tube-based detector technology that requires Anger logic to appropriately detect and localize the coincident annihilation photons. This is then processed using analog signal technologies. A recent technical advance that was predominately driven to enable clinical PET/MR was the introduction of solid-state, silicon-based photomultipliers with analog signal processing technologies. The most recent advance is the introduction of solid-state, digital photon counting (DPC) PET detectors and the replacement of analog signal processing, which enables a fully integrated digital detection and processing chain with markedly improved TOF timing resolution.
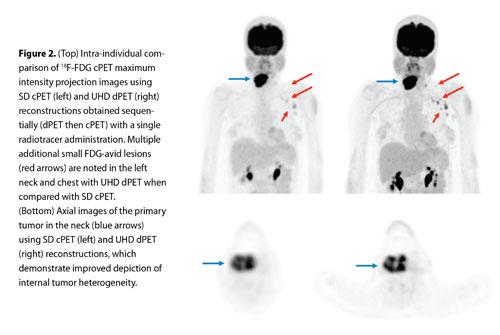
Our team is conducting pre-clinical and clinical trials using a pre-commercial release fully DPC-based PET (dPET) system that consistently provides 325 ps TOF timing resolution (Vereos Digital PET/CT, Philips). It also enables even higher-definition imaging using reconstruction voxel sizes of 1 mm3 with an ultra high-definition (UHD) reconstruction approach. Figure 2 presents an intra-individual comparison of a FDG whole-body PET scan performed back to back between a SD cPET and an UHD dPET.
Expanding Clinical Utilization
The current observations in the ongoing clinical trials with cPET and dPET already indicate that these new technologies and methodologies will significantly contribute to the goals of precision medicine and will further expand the clinical utilization of PET to be a clinically even more useful biomarker to guide the management of personalized oncologic therapies.
Michael V. Knopp, M.D., Ph.D., coordinating author, is professor of radiology and Novartis chair of imaging research, principal investigator and director, Wright Center of Innovation in Biomedical Imaging and the Ohio Imaging Research and Innovation Network. He is also vice chair of research for the Department of Radiology at The Ohio State University Wexner Medical Center.


 February 13, 2026
February 13, 2026 









