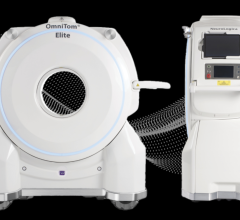
The Philips Ingenuity TF PET/MR system features separate PET and MRI scanners with one automated table gantry.
The new imaging modality of positron emission tomography (PET)/magnetic resonance imaging (MRI) was introduced in the U.S. market in 2011. The Siemens Biograph mMR (molecular MR) gained U.S. Food and Drug Administration (FDA) 510(k) clearance as the first dedicated PET/MRI system. Philips followed with 510(k) in November for its Philips Ingenuity TF PET/MR.
The Siemens system is the only system to combine both modalities into one machine, allowing simultaneous imaging of location, function and metabolic activity of organs in a single image.
“The Siemens PET/MRI system allows two tests to run simultaneously without having to move the patient to a different scanning system,” said Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostic Device Evaluation and Safety, FDA Center for Devices and Radiological Health. “Minimizing changes in a patient’s position between tests allows physicians to compare images more easily and helps them get the most accurate information possible.”
The Biograph mMR uses MRI rather than computed tomography (CT) to produce detailed images of the internal structures of the body. Because MRI makes images based mainly on the concentration of water in the body, it can produce greater detail of nearly all the internal structures of the body as compared with CT X-ray imaging. This may provide additional information about a patient’s condition.
Additionally, the Biograph mMR (molecular MR) system allows physicians to acquire images at a significantly lower radiation dose compared with a PET/CT system. Although the radiation dose from the PET exam remains unchanged, MRI does not use ionizing radiation, so the dose from the CT scan is eliminated.
In addition to Siemens, Philips Healthcare and GE Healthcare also developed PET/MRI systems. All three vendors took different approaches to how their systems operate and how they overcome the technical issues of combining PET in or near intense MRI magnetic fields.
Promising Future for New Modality
“PET/MR is likely to have a major impact in the future, as PET/CT did when it was introduced,” said Michael Graham, M.D., Ph.D., director of nuclear medicine, University of Iowa, during a press conference on key molecular imaging research at the 2011 Society of Nuclear Medicine (SNM) meeting. “I think we will see this technology proliferate over the next few years.”
He said it might be used to image complex soft tissue, such as the brain, head, neck and pelvis. It also will be very appealing for pediatric imaging to help reduce radiation dose from CT scans.
While MRI provides exquisite morphological and functional details in soft tissue, PET goes further to investigate the human body at the level of cellular activity and metabolism.
PET/MR is a tailored imaging modality for women and children, said Bruce Rosen, M.D., Ph.D., professor of radiology, Harvard Medical School, director of the Martinos Center of Biomedical Engineering at Massachusetts General Hospital. He said it eliminates CT radiation dose and that MRI is better at imaging soft tissue anatomy, such as female pelvic organs.
It also is ideal for oncology, because Rosen said MRI is good at imaging tumor angiogenesis, monitoring hypoxia to see if treatment is working. MRI allows tractography, imaging tracts of neuron activity to determine what is tumor and what is healthy brain tissue. It can measure blood-brain barrier permeability, too.
“Combining MRI technology with PET in a single integrated system adds the advantages of the extremely broad spectrum of diagnostic MRI procedures to the arsenal of available PET procedures,” said Alexander Drzezga, M.D., TU Muenchen, Munich, Germany, lead author of a study of the Siemens system, which he presented at the SNM meeting. “This could potentially result in the development of new imaging agents that bring together specific diagnostic strengths of PET and MRI.
“It offers exciting scientific options to image physiologic and pathophysiologic processes at the same time and to improve our understanding of both,” Drzezga added. “This and further studies could potentially open a whole new hybrid imaging discipline within the field of nuclear medicine.”
Siemens’ Approach
The Siemens Biograph mMR received European CE mark in the first week of June and FDA clearance the second week of June this year. Four machines are already installed at Massachusetts General Hospital in Boston under an FDA investigational device exemption (IDE).
Graham said the Siemens PET/MRI system is a major departure from previous PET systems. Traditional photomultiplier tubes could not be used because of the immediate proximity of the MRI magnets. Instead, Siemens uses avalanche photodiodes (APDs), new types of detectors that use ceramic instead of metal components.
Another development that helped facilitate a combined PET/MRI scanner was larger bore sizes. The 70 cm-bore MRI systems introduced in recent years provide the additional space needed to install the PET detectors, which reduces the bore size to about 60 cm.
The Siemens system allows completely simultaneous imaging of both anatomy and function, which cannot be done in either the Philips or GE solutions. This exact coordination in images may enable new avenues of clinical research, such as mapping brain response to stimuli.
Siemens representatives are quick to point out there is no time savings or benefit in using two separate devices and creating two separate imaging exams and then merging them. That type of exam can take 60 to 90 minutes, they say, whereas the Siemens mMR can do simultaneous imaging in about 30 minutes. They say it is similar to an MRI exam, except with the addition of an FDG injection for PET.
New types of artifacts will be encountered with PET/MRI systems due to the MRI coils. Siemens says using the coils built into the mMR table is not an issue, because the system’s software has already mapped these coils and will correct their image artifacts. But the use of movable coils may present artifacts that need to be corrected. This issue is overcome in the GE and Philips systems by separating the imaging systems.
Philips Approach
The Philips Ingenuity TF PET/MR gained European CE mark in January 2011 and gained FDA 510(k) clearance in November, with a system being used for investigational purposes at Mt. Sinai Hospital.
Philips Healthcare created a system that uses a separate PET and MRI scanner located in the same room. The innovation is the use of an automated table gantry. The system images the patient on the MRI and then rotates 180 degrees to image the patient in the PET scanner. The mobile table allows imaging of patients to be done without moving them from one table and scanner to another, which leads to misalignments on the merged hybrid images.
The system requires more space than a single MRI or PET scanning room, instead taking the space of between one-and-a-half to two rooms.
GE Healthcare’s Approach
Instead of developing a new, dedicated PET/MRI system, GE developed a single mobile patient table that is transferred between its PET/CT and MRI scanners for registered, tri-modality imaging. It maintains patient positioning between the two scanners for better registration by keeping the patient in the same position. The table loads into each scanner using a set of identical rails. The PET/MRI system is FDA-cleared. The GE systems combines the Discovery PET/CT + MR Discovery 710 and Discovery 3T 750w.
Vivek Bhatt, general manager, PET/CT, GE Healthcare, said GE took the approach of examining the clinical value of PET/MRI first, without spending a lot of time and money to develop a standalone system before a definite need has been established. He said the GE system offers a cost-effective solution that uses hospitals’ existing GE scanning equipment and allows clinicians to research the modality.
Gustav von Schulthess, M.D., Ph.D., department of medical radiology, University Hospital Zurich in Switzerland, has been using the GE PET/CT+MR solution to study its clinical value.
All three vendors are expected to show their new PET/MRI systems at the Radiological Society of North America (RSNA) meeting in Chicago.


 February 03, 2026
February 03, 2026