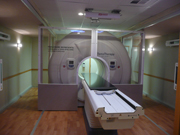
This view of a TomoMobile unit shows the Hi-Art treatment system housed inside.
Launching a new cancer center can be a long process, taking several months or even years to see a return on investment. But a new approach that includes the immediate introduction of radiation therapy services delivered in a relocatable unit is helping new cancer centers start building a patient base long before a permanent facility is complete.
This approach proved to be a viable solution for a cancer center in Mishawaka, Ind., where a relocatable radiation therapy solution was used to jumpstart operations at a new facility. Randy Christophel, executive vice president and chief operating officer, and Joe Gagliardi, senior vice president, of Goshen Health System and the Goshen Center for Cancer Care, are two of the executives who were involved in the project. In this article, they answer questions about how and why they decided to use this approach.
What was the decision process for the Goshen Center for Cancer Care to open a new cancer center in Mishawaka?
The patients and physicians in St. Joseph County, Ind., and the surrounding region were interested in a fully integrative, multidisciplinary approach to the evaluation and treatment of cancer patients. The hope was to include all oncology physician specialties, plus complementary services, in the same location in a coordinated and cohesive fashion.
In addition, physicians in the South Bend Clinic indicated their desire to raise the level of cancer care and services available in their market and contacted us to bring these capabilities to St. Joseph County. The new cancer center has been named Cancer Care Partners to reflect the partnership and combined efforts of the Goshen Center for Cancer Care and the South Bend Clinic.
From start to finish, how long does it typically take to open a new cancer center, and what challenges do you face?
It is hard to say what is “typical.” We spent approximately two years in the formation of our partnership with the South Bend Clinic and the design and initiation of the new cancer center. Some of the challenges that we encountered were:
- Designing and articulating the fully integrated, multidisciplinary model of care and inviting providers who can thrive and excel in this environment.
- Locating or constructing a facility that had sufficient space for all specialties and the various equipment and tools necessary to provide the level of care we desired. Typically, facilities like this don’t exist, nor are they available on the market for purchase or lease. After the decision to move forward, we began the long process of designing a facility to meet our needs.
- Recruiting additional physicians and other staff specialties.
How long did it take to complete the facility and begin serving patients?
We decided in late 2009 to create the new cancer center and began looking for locations and designing the facility. We became aware of a large medical clinic that was becoming available, but would need significant renovations and additions to the building to provide for our comprehensive cancer services.
For various competitive reasons, we wanted to fast-track the opening of the facility. We knew that the construction of the addition to the facility and the radiation vaults could not keep up with our desired timeline to begin providing services in August 2010. So, we decided to pursue the implementation of mobile services for positron emission tomography/computed tomography (PET/CT) and tomotherapy radiation therapy treatments for the first several months of the clinic’s operation. We felt this would allow time for facility buildout and implementation of fixed site equipment at a later date.
What types of treatments are offered at your permanent facility?
We are offering comprehensive cancer care, including surgical services, radiation therapy, chemotherapy, naturopathic and dietetic services, various counseling and support groups, and extensive patient education resources.
What made you consider using TomoMobile at your Goshen location?
We knew that the facility construction lead-time and the ordering timeline for a fixed unit would not meet our desire to open in August 2010. We also wanted to open the facility with full treatment capabilities, including tomotherapy treatments. So we began to pursue the TomoMobile unit for use on a temporary basis. That was one way we could begin to offer radiation therapy services before the construction of the addition and vaults were complete. TomoMobile consists of a standard TomoTherapy radiation therapy system housed in a custom-designed movable coach that replicates the environment of a conventional treatment vault.
Why did you chose to rent the TomoMobile solution on a temporary basis instead of purchasing it?
We rented the unit on a temporary basis to be able to provide radiation therapy services when we opened the facility in August. We could treat patients right away, while we continued construction of the radiation therapy vaults, where we will install a permanent TomoTherapy treatment system in 2011. We began treating patients within a couple of months of the decision to rent the unit.
How has the mobile unit impacted your business operations and your anticipated return on investment?
We just began providing services near the end of August 2010 and it allowed us to provide a full spectrum of radiation therapy services from the day that we opened the new facility. Being able to treat patients before the permanent facility is open has enabled us to accelerate our entire pro forma to be able to provide a full list of radiation therapy services from the start, rather than 12 to 18 months later.
The mobile unit also enabled us to treat patients on a joint modality basis without having to send them to other facilities. Our cancer program is successful because of its comprehensiveness and integration of services under one roof.


 March 06, 2026
March 06, 2026 









