The last decade has seen a significant advancement in imaging technology due to developments in the hardware and software space. It was clear to the radiologists, clinicians and imaging scientists very early on that no single imaging modality, be it magnetic resonance imaging (MRI), computed tomography (CT) or positron emission tomography (PET) could meet all the needs of a clinician treating a patient.
Moreover, on different systems, metabolic and structural images have to be acquired independently, requiring sequential patient examination and read-by-side, which makes the process error-prone. It became obvious that combining imaging modalities was the next step in advancing imaging technology. CT/PET imaging modality was one of the initial steps in that direction, combining the high speed of acquisition and high spatial resolution achieved with CT with high sensitivity and functional metabolic imaging capabilities achieved with PET.
Unfortunately, this technology had limitations, mainly for use in clinical trials where serial longitudinal imaging is necessary. Concerns around radiation exposure to subjects enrolled in clinical trials, combined with lack of sufficient tissue contrast (in some cases) resulted in the technology not being widely accepted as anticipated.
MRI has several advantages over CT, especially for use in clinical trials. These include the absence of ionizing radiation, which makes the modality safe for serial longitudinal imaging, accurate anatomical localization, good soft tissue contrast and the ability to image in multiple planes in a single setting. It is expected that clinical trials in the oncology, neurology and cardiovascular therapeutic areas will benefit the most from this hybrid technology.
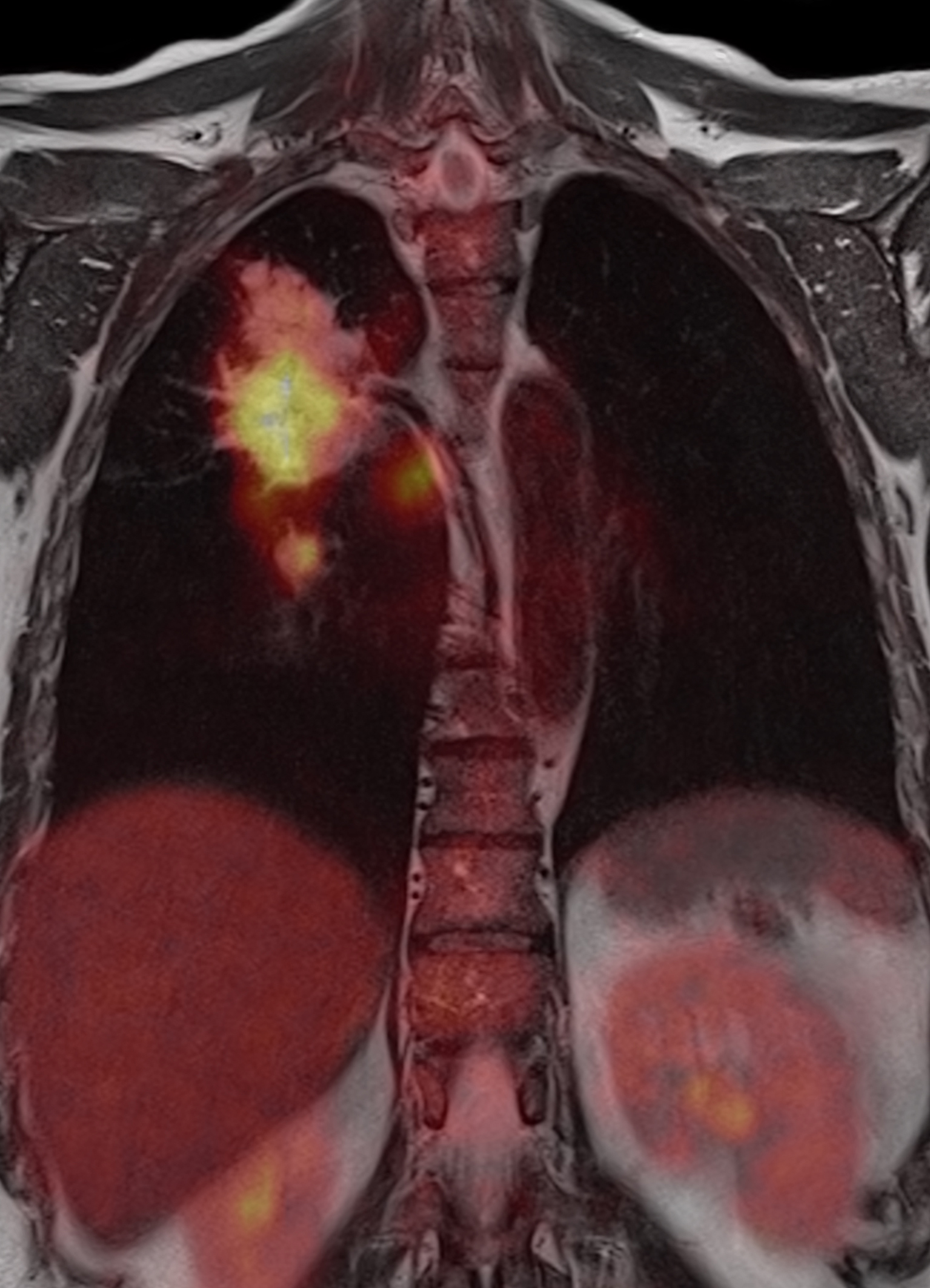
In oncology, the MRI component has proven beneficial for malignant diseases such as brain, breast, bone and head and neck tumors. In particular, MRI is the preferred modality for liver metastatic lesions because of its ability to detect small liver parenchymal lesions. Combining this technology with PET significantly enhances the sensitivity of detection of liver lesions and improves characterization of liver pathology. In a study published in the Nature medicine journal1, the authors reported success by demonstrating early detection of soft tissue tumors in a preclinical model, thereby paving way for an early therapeutic intervention using the hybrid technology.
MRI also offers the ability to measure additional parameters, such as tumor vascularization and perfusion properties, which enable intravascular treatment and monitoring of chemo-intervention. The dynamic contrast enhanced (DCE) MRI method allows assessment of vascular permeability in tumors, allowing longitudinal monitoring of response to antiangiogenic therapy.
MRI/PET hybrid scanners offer application to molecular imaging, which involves targeting individual receptors with radioligands, making them visible on PET and overlaying that information on high-resolution MR images for anatomical localization. Diffusion weighted imaging (DWI) offers special insight into diffusion changes in tissue resulting from ischemic disease and response to therapy, for example, in neuroendocrine tumors where PET provides hormone receptor expression information, complementing diffusion information from MRI.
Specifically in neurology, MR/PET systems offer significant benefits in Alzheimer’s disease, Parkinson’s disease, schizophrenia and epilepsy. In Alzheimer’s disease, MRI and PET already are the imaging modality of choice. For example, in Alzheimer’s disease clinical trials, imaging subjects on these scanners will allow clinicians and scientists to perform amyloid imaging and volumetric acquisitions, and co-register MR/PET images in real time without going through a computationally intensive post-processing co-registration workflow.
Additionally, since not all sites may have both MRI and PET technologies in one location, access to an MRI/PET hybrid technology would significantly streamline the logistics of scheduling scanner time for imaging trial subjects. In many cases, this translates to significant cost savings, particularly in large-scale late-phase clinical trials.
There is a great deal of emphasis on the development of quantitative imaging biomarkers that can help evaluate response to therapy in trials involving imaging. In some cases, imaging biomarkers extend beyond a single modality and thus, correlating them across scanners and sites becomes a challenge. The ability to obtain all of the data from a single scanner simplifies the task of validating and correlating quantitative biomarkers. For cardiovascular imaging, MR/PET technology allows improved assessment of myocardial perfusion, evaluation of viability of atherosclerotic plaques and coronary angiography.
However, in addition to the benefits of MR/PET, there are some deficiencies with this technology which will prevent CT/PET from becoming obsolete anytime soon. CT still remains the modality of choice for detection of small pulmonary nodules in lung malignancies, assessment of bone mineralization and for cases where MRI is contraindicated due to cardiac pacemaker and/or metallic implants. But despite some of these limitations, it is clear that MRI/PET offers several advantages over other modalities, including CT/PET, and truly has the potential to become the modality of choice in clinical trials. itn
Rohit Sood, M.D., Ph.D. is the senior medical director, head of advanced imaging and head of neuroimaging at Parexel. Parexel is a global bio/pharmaceutical services organization that helps clients expedite time-to-market through its development and launch services. These include a range of clinical development capabilities, integrated advanced technologies, regulatory affairs consulting and commercialization services.
Reference:
1 Judenhofer, M. Simultaneous PET-MRI: A new approach for functional and morphological imaging. Nature Medicine 2008:14; 459-465.


 February 13, 2026
February 13, 2026 









