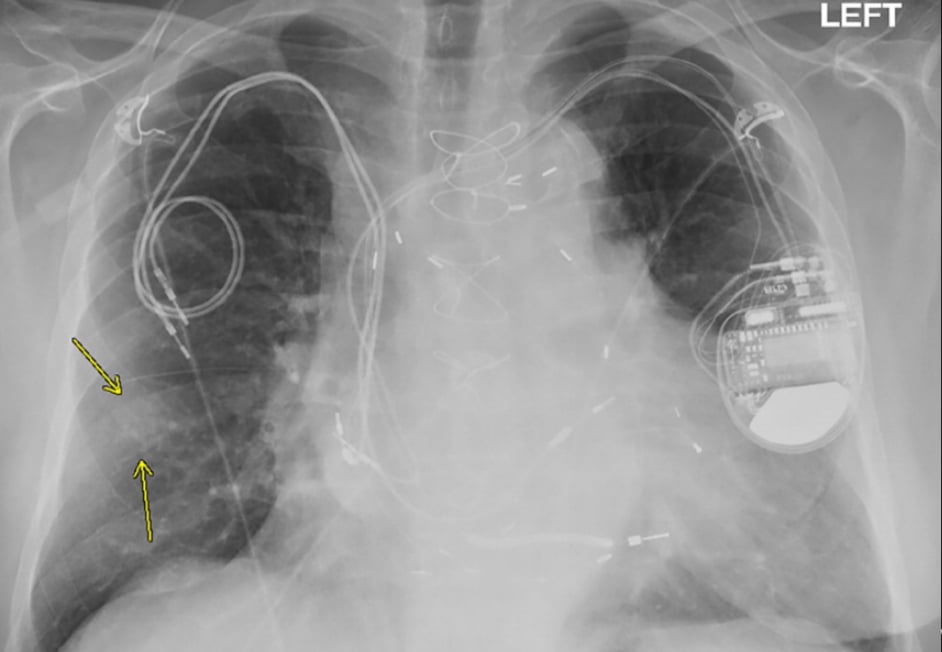
Chest X-ray from a patient included in the study. Posteroanterior view, of a 79-year-old man with history of a previous pacemaker, with abandoned right atrial and right ventricular pacing leads on the right side at time of new cardiac resynchronization therapy defibrillator implant on the left side. Arrows indicate a nodular opacity in the right midlung concerning for mass. Find more images of patients in this study in Radiology: Cardiothoracic Imaging.
October 27, 2020 – Magnetic resonance imaging (MRI) examinations can be safely performed in patients with non-MR compatible cardiac devices, including pacemakers, abandoned pacing leads, and implantable cardioverter defibrillators (ICDs), according to a new study published in Radiology: Cardiothoracic Imaging.[1]
Millions of people around the world rely on implanted cardiac devices like pacemakers and ICDs to help control abnormal heart rhythms. MRIs are discouraged or prohibited in many of these people over fears that the powerful magnet of the scanner will heat the metal in the devices, potentially damaging heart tissue and harming the devices.
The U.S. Food and Drug Administration (FDA) has identified a subset of implanted cardiac devices as MR conditional, meaning they pose no known hazard under specified conditions. Pacemakers or ICDs that have not met the criteria are considered non-MR conditional. In these patients, clinicians are forced to choose between removing a necessary cardiac device or getting an alternate and potentially less-effective imaging test.
“While all devices implanted in patients today are MRI compatible, millions of people worldwide, including many young people, have older devices considered not compatible,” said study lead author Sanjaya K. Gupta, M.D., an electrophysiologist from Saint Luke’s Mid America Heart Institute in Kansas City, Mo. “It’s unfair to tell these people that they can’t get an MRI for the rest of their lives.”
Prior research has demonstrated the safety of performing MRI exams in patients with non-MR conditional devices. However, those studies did not account for pacemaker-dependent ICD patients, or patients whose hearts won’t function if the defibrillator is removed or stops working. Other groups not accounted for in previous studies include patients undergoing chest and cardiac MRI exams and patients with abandoned or fractured leads, or wires that connect the device to the heart.
To develop a more comprehensive picture of risk, Gupta and colleagues established the Patient Registry of Magnetic Resonance Imaging in Non-Approved Devices (PROMeNADe). They enrolled more than 500 participants who had undergone a total of 608 MRI exams, including 61 cardiac MRI exams. Participants included people from groups not accounted for in previous research.
Patients had their devices checked before and after each MRI and had their vital signs monitored closely by a nurse during their time in the scanner. Devices were turned to asynchronous mode in pacemaker-dependent patients before they went in the scanner. ICD patients had tachycardia therapies disabled during the MRI.
The results demonstrated that MRI exams—including chest MRI exams—can be performed safely in pacemaker-dependent ICD patients and in patients with non-MR conditional devices or abandoned leads.
“There were no adverse events,” Gupta explained. “The protocol worked amazingly well. We had no issues with any of the patients and no harm to the devices.”
The registry is now the largest series of MRI scans that has ever been put together in patients with pacemaker-dependent ICDs. It is also the second largest in patients with abandoned or fractured leads and the third largest in non-compatible devices.
Results from a survey of the physicians who had referred the patients for MRI underscored the importance of the exams. According to responses from 150 physicians, MRI results changed the suspected diagnosis 25% of the time and changed suspected prognosis in 26% of participants, with planned medical or surgical treatment being changed 42% of the time.
“We’re hopeful that our work will add support to expand the FDA’s indications for devices that are considered MRI-compatible,” Gupta said.
The study was inspired, in part, by the case of a patient with a brain tumor who was unable to get sorely needed MRI examinations because her pacemaker was not MR conditional. Without MRI results to precisely localize the tumor, the patient had to undergo several risky surgeries.
“A lot of work went into this study but it’s worth it when you consider all the lives impacted,” Gupta said. “I feel like it makes a difference to a lot of people.”
Related Content with Dr. Sanjaya Gupta:
VIDEO: Artificial Intelligence to Automatically Risk Stratify Atrial Fibrillation Patients — Interview with Sanjaya Gupta, M.D.
Artificial Intelligence Applications in Cardiology
Reference:


 February 13, 2026
February 13, 2026 









