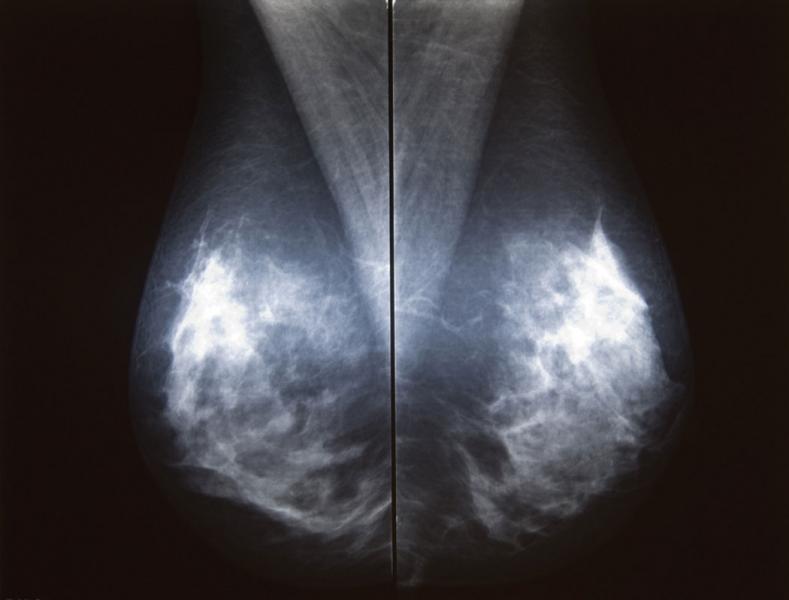
Mammomat Fusion is the latest addition to the Siemens family of full-field digital mammography systems that offers premium product features to address the specific needs of volume screening centers and small to medium-sized hospitals.
The outlook of breast cancer is changing, and death rates have been decreasing since 1989, thanks in part to treatment advances, earlier detection through screening and increased awareness. However, more than 40,000 women in the United States are still expected to die from this disease in 2015. According to the American Cancer Society, an estimated 231,840 new cases of invasive breast cancer are expected to be diagnosed in women in the United States this year, along with 60,290 new cases of carcinoma in situ (CIS). And for men, more than 2,000 new cases of invasive breast cancer are projected.
A national poll, conducted by the Truven Health Analytics-NPR Health Poll,1 has found that the majority of American women — 57 percent — still are in favor of an annual mammogram, despite the recent recommendation for biannual screenings issued by the U.S. Preventive Services Task Force (USPSTF) that suggested women should get a mammogram every two years starting at age 50, provided they don’t have a family history of breast cancer or find a lump. Only 12 percent of respondents said they believe the screening is only necessary every two years, as recommended by the USPSTF.
“The proper evaluation and treatment of localized breast cancer is an area of active research. The U.S. Preventive Services Task Force recommends biennial breast cancer screening with mammography in all average risk women between the ages of 50-74,” said Michael Taylor, M.D., chief medical officer at Truven Health Analytics. “With the Affordable Care Act mandating that insurers provide screening mammograms at no additional charge, patients have no reason not to be diligent about receiving a regular screening.”
On the Legislative Front
The American College of Radiology (ACR) and the Society of Breast Imaging (SBI) are encouraging congressional leaders to pass the Protecting Access to Lifesavings Screenings (PALS) Act (H.R. 3339).2 Passage of the act would ensure women who want regular mammograms retain insurance coverage with no co-pay and avert thousands of unnecessary deaths.
Both organizations support the PALS Act3 as a way to delay implementation of draft breast cancer screening recommendations from the USPSTF for two years. “The two-year delay allows consideration of recent large studies that showed mammography to be far more effective than the old studies the USPSTF analyzed. It also provides time for Congress to enact separate legislation that mandates a badly needed overhaul of the closed and outdated USPSTF process,” said Debra Monticciolo, M.D., FACR, chair of the American College of Radiology Breast Imaging Commission.
The Affordable Care Act (ACA) requires private insurers to cover exams without patient cost sharing given a grade of “B” or higher by the USPSTF. The Task Force gave routine screening of women ages 40-49 a “C” grade and gave a “B” grade only to biennial screening for women 50-74. This would indicate that women ages 40-49 that choose routine screening and those 50-74 who want annual screening would not be guaranteed coverage, which could have an impact in underserved and rural areas.
“The closed USPSTF process does not meet Institute of Medicine (IOM) standards for ‘trustworthy’ guidelines creation and needs updating. These USPSTF mammography recommendations are suspect until ACR and SBI recognized experts are included in a meaningful way in their creation,” said Elizabeth A. Morris, M.D., FACR, president of the Society of Breast Imaging.
The Breast Density Movement
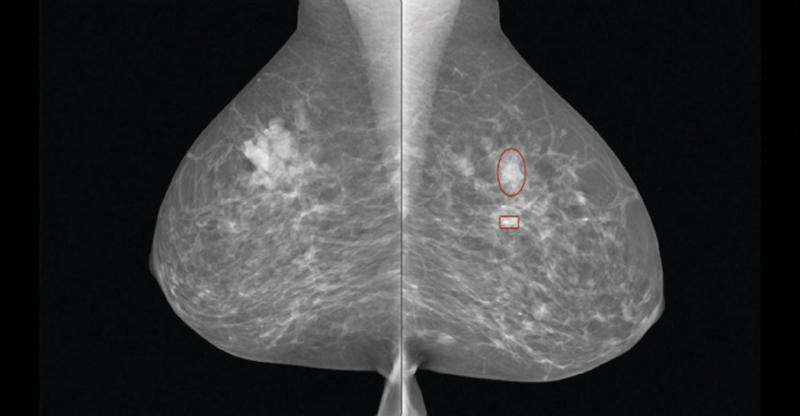
Louisiana recently became the 24th state to sign the density reporting bill into law. According to areyoudenseadvococy.org, additional screening tests beside mammography for women with dense breast tissue will increase detection by up to 100 percent. These invasive cancers, missed by mammography, are small, node negative and at an early stage.
“Universal density reporting will prevent later-stage cancers and give all women access to an early diagnosis — when most treatable and with better survival outcomes. Data show a statistically significant increase in the detection of small, early and invasive cancers invisible by mammogram,” according to Nancy M. Cappello, Ph.D., executive director and founder of Are You Dense Advocacy Inc.
States with breast density inform laws in order of enactment include Connecticut, Texas, Virginia, New York, California, Hawaii, Maryland, Tennessee, Alabama, Nevada, Oregon, North Carolina, Pennsylvania, New Jersey, Arizona, Minnesota, Rhode Island, Massachusetts, Missouri, Ohio, Michigan, North Dakota, Delaware and Louisiana.
Advances in Technology
Of course, detection is only possible through screening and technology, and this past year brought forth many technological advancements, helping to bring the issue of breast screening, and in particular breast density, to the forefront.
The U.S. Food and Drug Administration (FDA) recently approved two systems from Siemens Healthcare. Mammomat Fusion is the latest addition to the Siemens family of full-field digital mammography systems that offers premium product features to address the specific needs of volume screening centers and small to medium-sized hospitals. The system features a new generation cesium-iodide detector — an innovative, layered configuration of the photo diodes within the detector that enables more efficient utilization of the radiation dose. The result is a high image quality at a patient dose that is at or below the range of other full-field digital mammography systems, with an even lower dose delivered in cases where the patient’s breast thickness exceeds 50 mm. The system’s large image matrix of 23 x 30 cm makes it convenient for screening various breast sizes.
The FDA also approved Siemens’ 3-D mammography imaging system with breast tomosynthesis option, marking its tomo debut in the U.S. market. Siemens’ Mammomat Inspiration with Tomosynthesis Option is a breast tomosynthesis add-on option for Siemens Mammomat Inspiration digital mammography platform. Its breast tomosynthesis algorithm reconstructs multiple 2-D images of the breast into an approximation of a 3-D image to enable detection of tumors that are hidden by overlapping breast tissue. This helps to provide a more accurate diagnosis than standard 2-D digital mammography alone and helps to reduce the number of false-positive findings. In tomosynthesis mode, the X-ray tube of the Mammomat Inspiration rotates in a circular motion around the breast to acquire an image every two degrees while moving through an angular range of 50 degrees. The resulting 25 projections are reconstructed as
three-dimensional digital breast tomosynthesis (DBT) images.
iCAD Inc. launched the iReveal breast density module as the latest addition to its PowerLook Advanced Mammography Platform (AMP). The software is designed to deliver automated, rapid and reproducible assessments of fibroglandular breast density to help identify patients that may experience reduced sensitivity to digital mammography due to dense breast tissue. It uses an advanced software program to assess breast density based on the range of categories established by the American College of Radiology’s Breast Imaging Reporting and Data System (BI-RADS), automating the same analytical approach used by many experienced radiologists. In many cases, patients with dense breast tissue may be recommended for additional screening exams including use of breast tomosynthesis.
Fujifilm Medical Systems U.S.A. Inc. recently submitted to the FDA the first module of its premarket approval (PMA) application for DBT. The module will be offered as an optional upgrade for the Aspire Cristalle mammography system. Fujifilm plans to file the remaining modules of DBT PMA within the coming year. The optional DBT upgrade for the Aspire Cristalle system, known as Amulet Innovality outside of the United States, has been available since May 2013 in Europe, Asia and Latin America. Aspire Cristalle features Fujifilm’s hexagonal close pattern (HCP) detector pixel design, engineered for higher acquisition efficiency and to enhance detail for improved low-dose performance compared to conventional square pixel design. The result is sharper images with gentler dose to the patient.
Eizo Inc. has received FDA 510(k) clearance for breast tomosynthesis on its 5-megapixel monochrome medical monitor, the RadiForce GX540. The FDA 510(k) clearance includes tomosynthesis, mammography and general radiography. The monitor’s high resolution makes it ideal for viewing the fine details in breast images. To detect the smallest structures, the monitor offers a high contrast ratio of 1,200:1. The deeper black levels distinguish similar shades of gray for sharper monochrome image reproduction.
Novarad has developed its own specialty viewer with advanced hanging protocols and workflows designed especially for digital mammography. While Novarad customers have been using a third-party mammography viewer for years, the new viewer is completely native to the NovaPACS enterprise viewer. The new NovaMG offers radiologists the option to use their picture archiving and communication system (PACS) workstation to read solely mammography and tomosynthesis studies, or the convenience to read for all patients and all modalities from the same workstation and user interface.
The U.S. Patent and Trademark Office has issued a patent for the technology incorporated into Kubtec’s Mozart System with TomoSpec Technology. The system allows surgeons to bring 3-D tomosynthesis imaging directly into the operating room for lumpectomy and partial mastectomy procedures, and gives surgical teams the ability to make faster and more precise intraoperative determinations of the successful excision of tumor margins.
Community Outreach
Education and accessibility play a crucial role in the early detection and treatment of breast cancer. Many organizations have found unique ways to spread public awareness of and access to this cause.
For example, Loyola Medicine in Maywood, Ill., conducted a very successful See, Test & Treat Free Cancer Screening event that was funded by the College of American Pathologists (CAP) Foundation. This program is available at select institutions around the United States, however it was Loyola’s first.
The program helps vulnerable women in underserved communities improve their health. In September, more than 50 women in need received free cervical and breast cancer screenings with same-day results as part of Loyola’s program and health fair held at Loyola University Medical Center. This event was also supported by Hologic Inc., the Community Memorial Foundation, the Coleman Foundation and Quest Diagnostics.
“Many of the women we cared for had not had a Pap test or mammogram in more than 20 years, and thanks to the generous funding by the CAP Foundation, were able to receive care,” said Loyola Chair of Pathology Eva M. Wojcik, M.D., FCAP. “Seeing those signs of relief on the faces of women who were told their screenings came back normal was priceless.”
Future Outlook
The outlook for finding and dealing with breast cancer has improved dramatically in recent years, and will continue to improve exponentially, according to Jim Culley, Ph.D., senior director, corporate communications for Hologic.
Culley cites a recent study by Sarah Friedewald, M.D., published in the June 2014 issue of JAMA that looked at nearly 500,000 screening exams, some utilizing 2-D mammography and some using tomosynthesis.4 The objective of the study was to determine if mammography plays a key role in early breast cancer detection, noting that single-institution studies have shown that adding tomosynthesis to mammography increases cancer detection and reduces false-positive results.
Culley called out some of the study’s most significant findings:
• A 41 percent increase in the detection of invasive breast cancers (p<.001);
• A 29 percent increase in the detection of all breast cancers (p<.001);
• A 15 percent decrease in women recalled for additional imaging (p<.001);
• A 49 percent increase in positive predictive value (PPV) for a recall (p<.001). PPV for recall is a widely used measure of the proportion of women recalled from screening that are found to have breast cancer. The PPV for a recall increased from 4.3 to 6.4 percent;
• A 21 percent increase in PPV for biopsy (p<.001). PPV for biopsy is a widely used measure of the proportion of women having a breast biopsy that are found to have breast cancer. The PPV for a breast biopsy increased from 24.2 to 29.2 percent; and
• No significant change in the detection of ductal carcinoma in situ (DCIS). DCIS is a non-invasive cancer. It has not spread beyond the milk duct into any normal surrounding breast tissue.
“In the U.S., almost no one is buying 2-D systems that can’t be upgraded to 3-D anymore,” said Culley. “And if they can afford it, most new purchases are for 3-D mammography.”
The study concluded that the addition of tomosynthesis to digital mammography was associated with a decrease in recall rate and an increase in cancer detection rate. It was determined that further studies are needed to assess the relationship to clinical outcomes.
Culley concludes that it’s best to compare apples to apples, and agrees that the USPSTF guidelines are slightly out of touch with today’s technology. “There has been so much improvement that you can’t help but question the USPSTF and their new guidelines that look at long series of data to justify their recommendations,” he said. “These organizations haven’t really adjusted for the fact that technology is changing dramatically. In breast cancer screening no one does film mammography anymore, everyone does digital and since 2011, most are trying to use 3-D mammography.”
He gives the analogy of the advent of the digital camera. “Just think about the picture clarity of your phone, which was probably none, or digital camera back in 2000, and how much better images are now. It’s unreasonable to consider mammography today as it was then.”
References:
1 http://truvenhealth.com/truven-health-insights/health-insights/npr-poll. Accessed Sept. 21, 2015.
2 https://www.congress.gov/bill/114th-congress/house-bill/3339. Accessed Sept. 21, 2015.
3 https://www.govtrack.us/congress/bills/114/hr3339. Accessed Sept. 21, 2015.
4 http://jama.jamanetwork.com/article.aspx?articleid=1883018. Accessed Sept. 21, 2015.



 February 18, 2026
February 18, 2026 









