Coronary artery disease (CAD) is a major cause of death in modern industrialized countries. Assessments of regional myocardial perfusion at rest and during stress (exercise or pharmacologic coronary vasodilation) have proved valuable for noninvasive diagnosis of CAD. Myocardial perfusion imaging (MPI) with positron emission tomography (PET) has been shown to be superior to single photon emission computed tomography (SPECT). Nevertheless, widespread clinical use of PET MPI has been limited by the currently available PET myocardial perfusion tracers.
While the tracers N-13 labed ammonia (13NH3) and O-15 labeled water (H215O) can be used for MPI, they require an on?site cyclotron, and thus are impractical for widespread utilization. Rubidium-82 (Rb) may be prepared on site using a generator, but it is not an ideal PET tracer due three factors:
1. The high recurrent cost of the generator
2. The long positron range that lowers image resolution
3. A very short 75-second half-life that makes it incompatible with exercise-stress imaging.
Furthermore, the myocardial extraction fraction of Rb-82 is the lowest among the currently available PET perfusion tracers. This reduces the intensity of imaged perfusion defects and makes Rb-82 less than ideal for absolute quantification of myocardial blood flow (MBF).
Flurpiridaz F-18 is a new PET MPI radiopharmaceutical in clinical development. It is a structural analog of pyridaben and binds to mitochondrial complex I with high affinity.[1] Preclinical studies showed that the extraction fraction of flurpiridaz F-18 was greater than 90 percent.[1,2] The positron range of F-18 is approximately seven times shorter than that of Rb-82,[1] so it would be expected to produce images with higher resolution. With a longer half-life, 18F?labeled flurpiridaz may be produced at regional cyclotrons and delivered to imaging centers in much the same way as F-18 labeled fluorodeoxyglucose (FDG), thus obviating the need for an on-site cyclotron. The longer half-life of F-18 also ensures that the radiotracer is present long enough to allow a patient injected at peak treadmill exercise to move to the camera and still be effectively imaged. Higher myocardial extraction facilitates detection of milder perfusion defects[5] and allows more accurate quantification of MBF.
Clinical Studies
Phase 1 and Phase 2 clinical studies have been completed with flurpiridaz F-18, and Phase 3 studies are currently ongoing.
The objectives of the Phase 1 investigations (Studies 101 and 102) were to assess the safety, tolerability, dosimetry, and biodistribution of flurpiridaz F-18 in healthy subjects. In Study 101,[6] the mean effective dose (ED) of flurpiridaz F-18 injected at rest was very similar to that of the FDG, with a much lower exposure to the critical organ by a factor of 2.5. High myocardium-to-background uptake ratios and an encouraging safety profile were noted.[10]
In Study 102,[7] the investigators examined the safety, dosimetry, biodistribution, and myocardial imaging characteristics of flurpiridaz F-18 injected at rest and on a second day, at peak adenosine stress (n=6) or at peak treadmill exercise (n=6). Excellent image quality was noted with both forms of stress imaging. Dosimetry results suggest that injection of up to 14 mCi of flurpiridaz F-18 during a rest-stress protocol would provide a clinically acceptable ED. As in Study 101, no adverse events related to flurpiridaz F-18 were noted.
The objectives of the Phase 2 trial[8] were to assess flurpiridaz F-18 for safety and compare its diagnostic performance for PET MPI to technetium (Tc)-99m SPECT MPI regarding image quality, interpretative certainty, defect magnitude and detection of coronary artery disease (CAD)(?50% stenosis) on invasive coronary angiography (ICA). The study included 143 patients from 21 centers who underwent rest-stress PET and Tc-99m SPECT-MPI. Eighty-six patients underwent ICA, and 39 had low-likelihood of CAD. Images were scored by three independent, blinded readers.
A higher percentage of images were rated as excellent/good on PET vs. SPECT on stress (99.2 vs. 88.5%, p<0.01) and rest (96.9 vs. 66.4, p<0.01) images. Diagnostic certainty of interpretation (percentage of cases with definitely abnormal/normal interpretation) was higher for PET vs. SPECT (90.8 vs. 70.9%, p<0.01). In 86 patients who underwent ICA, sensitivity of PET was higher than SPECT [78.8 vs. 61.5%, respectively (p=0.02)]. Specificity was not significantly different (PET 76.5% vs. SPECT 73.5%). Receiver operating characteristic curve area was 0.82±0.05 for PET and 0.70±0.06 for SPECT (p=0.04). In patients with CAD on ICA, the magnitude of reversible defects was greater with PET than SPECT (p=0.008). Extensive safety assessment revealed that flurpiridaz F-18 was safe in this cohort.
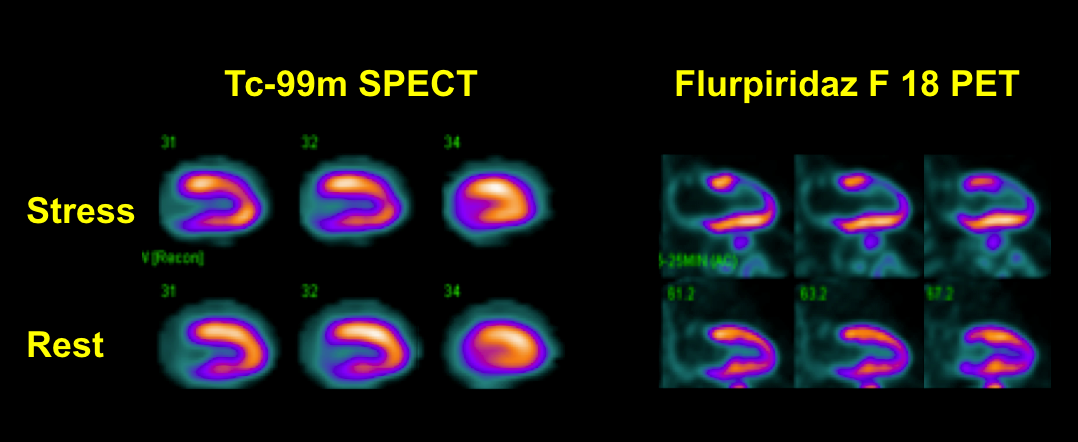
Figure 1 demonstrates SPECT and PET images in a patient with significant disease in the left anterior descending coronary artery. The overall quality of the PET images (lower rows) was superior to the Tc-99m SPECT images (upper rows). Flurpiridaz F-18 PET images showed severe anterior and apical defects in the distribution of the diseased LAD coronary artery, but the Tc-99m SPECT images showed only a small and mild antero-apical defect.
Figure 2 shows Tc-99m SPECT images (upper rows) and flurpiridaz F-18 PET images (lower rows) from a patient with a low likelihood of CAD. A false positive partially reversible inferior defect is present on the Tc-99m SPECT images due to soft-tissue attenuation. The flurpiridaz F-18 PET study, however, provided superior image quality and was normal.
Absolute Quantification of Myocardial Blood Flow
The preliminary results of absolute quantification of myocardial blood flow with flurpiridaz F-18 PET represents a major advantage over SPECT MPI, and is a potential game-changer in the noninvasive evaluation of CAD. Studies with 13NH3, H215O, and Rb-82 have shown that absolute quantification of MBF allows better identification of multivessel disease, assessment of the severity of microvascular disease, allows evaluation of endothelial dysfunction and responses to treatment, and has incremental value in prognostication in patients with suspected or known CAD.[9] The authors developed a model for quantification of absolute MBF using flurpiridaz F-18.[10] CFR in LL patients was 3.7+ 0.39, similar to that expected physiologically. However, mean stress MBF (induced with adenosine) and CFR were significantly lower in myocardial regions supplied by diseased coronary arteries.
Data from these and the Phase 3 pivitol trial, once completed, will be used for submission to the FDA.
Editor’s note: The second Phase 3 trial for flurpiridaz F-18 will likely commence in 2014. The filing of the FDA new drug application is dependent on the time it takes to fully enroll and complete the trial.
The authors Jamshid Maddahi, M.D., FACC, FASNC, and René R. S. Packard, M.D., are from the departments of molecular and medical pharmacology (nuclear medicine) and medicine (cardiology) at the David Geffen School of Medicine at University of California, Los Angeles (UCLA).
References:
1. Yalamanchili P, Wexler E, Hayes M, Yu M, Bozek J, Kagan M, et al. “Mechanism of uptake and retention of F-18 BMS-747 158-02 in cardiomyocytes: a novel PET myocardial imaging agent.” J Nucl Cardiol 2007; 14:782-788.
2. Huisman MC, Higuchi T, Reder S, Nekolla SG, Poethko T, Wester H-J, et al. “Initial characterization of an 18F-labeled myocardial perfusion tracer.” J Nucl Med 2008;49:630?6.
3. Nekolla SG, Reder S, Saraste A, Higuchi T, Dzewas G, Preissel A, et al. “Evaluation of the novel myocardial perfusion PET tracer 18F-BMS747158-02: Comparison to 13N?ammonia and validation with microspheres in a pig model.” Circulation 2009;119:2333-42.
4. Garcia EV, Galt JR, Faber TL, Chen J. “In: Atlas of Nuclear Cardiology (3rd edition),” Vasken Dilsizian & Jagat Narula (eds). Current Medicine LLC, Chapter 1, pp 1-34, 2009.
5. Maddahi J. “Properties of an ideal PET perfusion tracer: new PET tracer cases and data.”
J Nucl Cardiol. 2012 Feb;19 Suppl 1:S30-7.
6. Maddahi J, Czernin J, Lazewatsky J, Huang S-C, Dahlbom M, Schelbert H, et al. “Phase I, first-in-human study of BMS747158, a novel 18F-labeled tracer for myocardial perfusion PET: Dosimetry, biodistribution, safety, and imaging characteristics after a single injection at rest.” J Nucl Med 2011;52:1490-1498.
7. Maddahi J, Bengel F, Huang S-C, Czernin J, Schelbert H, Zhu Q, et al. “Phase 1 rest?stress study of F-18 labeled BMS747158 myocardial perfusion PET tracer: Human safety, dosimetry, biodistribution, and myocardial imaging characteristics [abstract].” J Nucl Med 2009;50:184.
8. Berman DS, Maddahi J, Tamarappoo BK, Czernin J, Taillefer R, Udelson JE, Gibson CM, Devine M, Lazewatsky J, Bhat G, Washburn D. “Phase II safety and clinical comparison with single-photon emission computed tomography myocardial perfusion imaging for detection of coronary artery disease: flurpiridaz F 18 positron emission tomography.” J Am Coll Cardiol. 2013 Jan 29;61(4):469-77.
9. Schelbert HR. “Quantification of myocardial blood flow: What is the clinical role? Cardiol Clin 2009;27:277-89.
10. Maddahi J, Huang S, Truong D, Lazewatsky JL, Ehlgen A, Schelbert H, et al. “Preliminary results of absolute quantification of rest and stress myocardial blood flow with flurpiridaz F-18 PET in normal and coronary artery disease patients in a single-center study [abstract].” J Nucl Cardiol 2010;17:743.