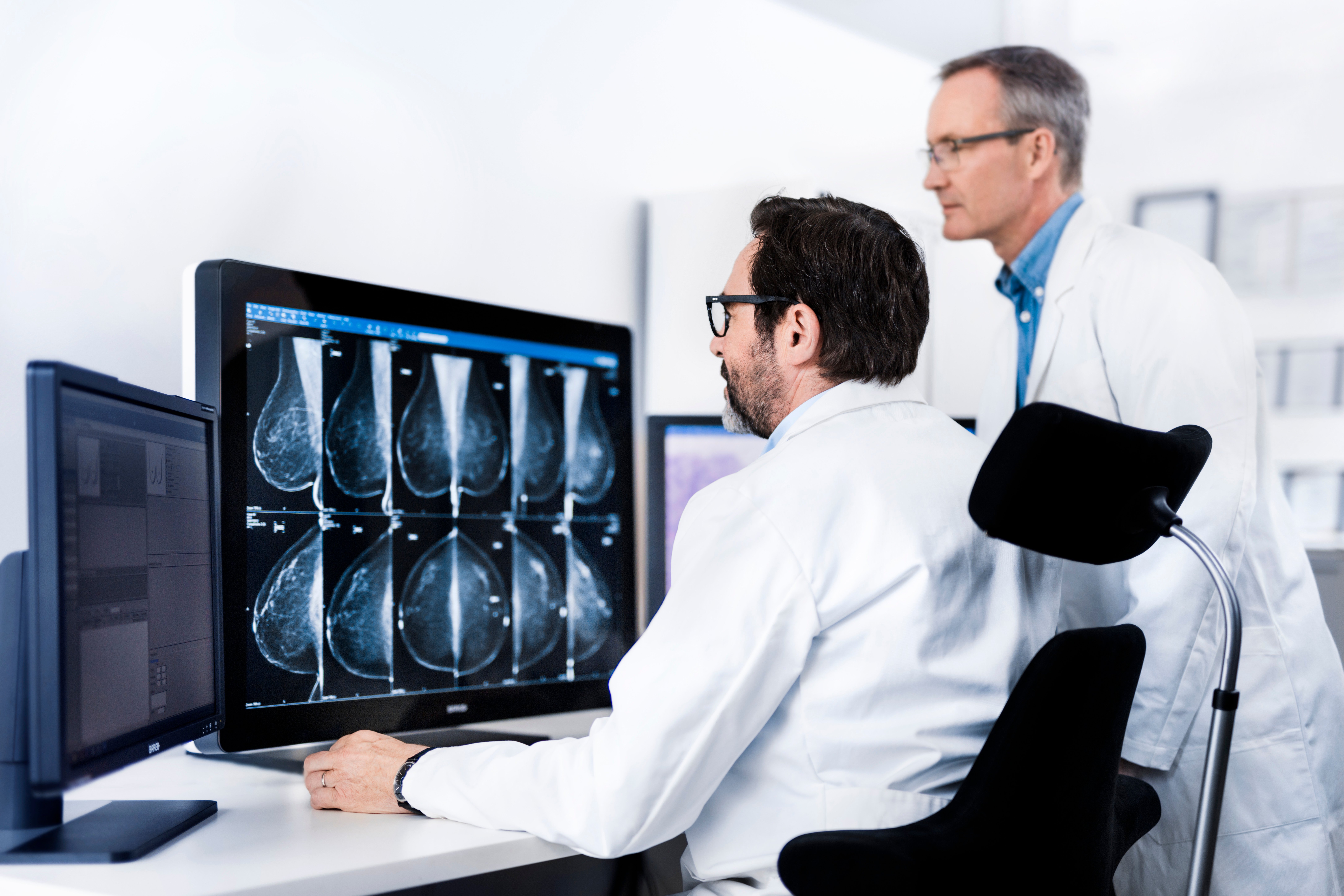
February 8, 2017 — The U.S. Food and Drug Administration (FDA) has released a new document detailing best practices for retaining patient mammography records in response to ongoing confusion from some mammography facilities, according to the agency.
According to the FDA, the Mammography Quality Standards Act (MQSA) includes provisions related to record retention and transfer. Under MQSA, original mammograms and their reports are considered medical records. A facility must “maintain mammography films and reports in a permanent medical record of the patient for a period of not less than five years, or not less than 10 years if no additional mammograms of the patient are performed at the facility, or a longer period if mandated by State or local law” (900.12(c)(4)(i)), according to FDA. This requirement to retain prior exams facilitates the comparison of a patient’s more recent exams to her earlier exams, since assessing for stability or change over time can be a very important aspect of mammographic interpretation, the agency said.
Under MQSA, the length of time that a particular mammogram must be retained depends on both the date of that exam and the date (if any) when the patient last returned to the facility. At a minimum, each mammogram must be retained for at least five years, the FDA document said. If a patient has returned to the facility within those five years for one or more subsequent mammograms, only the most recent five years’ worth of exams must be retained. If a patient has not returned for another mammogram at the facility within five years of her most recent exam performed there, the facility cannot know when she might return for another exam (or might request her exams), so her mammograms must be retained for up to five additional years (10 years in total) in case she returns or makes such a request, according to the FDA.
However, the agency said to remember that states may impose more stringent requirements for medical record retention, and facilities must also comply with any applicable state or local requirements. Also, the MQSA record retention requirement is a baseline standard. For continuity of care, if a patient is returning regularly for subsequent exams, many facilities opt to retain all of that patient’s mammograms, not only those exams which are less than five years old.
The agency said it is important to note that there is no difference in the required length of time for retention of digital mammograms compared to screen-film mammograms. Screen-film mammograms should be retained in their original form, neither as copy films nor digitized. The Policy Guidance Help System advises that if a mammogram was originally produced in digital form, such as full-field digital mammography (FFDM) or digital breast tomosynthesis (DBT), it should be retained in a fully retrievable form, either as an uncompressed digital image, or using only lossless (not lossy) data compression, or it may be retained as a hard-copy image of final interpretation quality. (This latter option may not be practical for DBT exams.)
Another provision of MQSA addresses the transfer of mammograms. Mammograms may be needed for diagnosis or treatment somewhere other than the facility where the mammograms were performed. Therefore, protecting a patient’s access to her mammograms is important to the continuity of patient care, the agency said. In the era of screen-film mammography, it was especially important to transfer the original mammograms, rather than copies which might be of lesser quality. Therefore, under MQSA regulations, written when screen-film mammography was the mammographic modality in clinical use, a facility must “upon request by, or on behalf of, the patient, permanently or temporarily transfer the original mammograms and copies of the patient's reports to a medical institution, or to a physician or healthcare provider of the patient or to the patient directly” (900.12(c)(4)(ii)).
In the current era of digital mammography (FFDM and DBT), the actual transfer of the original digital mammogram is rare. Instead, digital copies of mammograms are usually released, while the facility retains the originals. Since this is a release of copies, the transfer provision of MQSA does not apply, according to FDA.
Off-site storage of medical records is permitted under MQSA. There is no requirement that the storage of retained records be within the facility itself, and mammograms and reports do not necessarily need to be stored together at the same location, the FDA said. Whether records are stored on-site or off-site, however, it is important to make contingency plans to prevent the loss of records due to disaster or equipment failure, such as the failure of a picture archiving and communication system (PACS). As discussed in a previous MQSA Insights article, if your PACS fails and images are lost due to preventable reasons, the FDA may take one or more actions against your facility. Remember, there may also be applicable requirements for contingency planning for record retention under the Health Insurance Portability and Accountability Act (HIPAA) and/or state or local laws, and facilities must comply with all applicable requirements.
Finally, according to the document, there are MQSA recommendations for the disposition of medical records (mammograms and reports) if a facility closes or ceases performing mammography. The facility should (a) inform its accreditation body that it will no longer be performing mammography; (b) notify its state radiation control program; (c) arrange transfer of each patient’s medical records to the facility where the patient will receive future care, to the patient’s referring provider or other provider designated by the patient, or to the patient or her representative; and (d) inform the patient of the arrangements made. Facilities should also check with state or local agencies to determine whether other requirements apply, the document said.
If transfer of all records is not feasible, facilities may store the remaining records in a hospital, other healthcare facility or a warehouse, but should assure that there is a mechanism to release records to an appropriate entity when requested, and should make patients aware of that mechanism. Facilities that remain open but have permanently ceased performing mammography may choose to keep patient medical records rather than transfer them to another facility (unless a patient requests such a transfer).
Because some facilities have not followed these recommendations, the agency said patients have contacted FDA with concerns about where and how to request their records. Therefore, the agency also requests that a closing facility notify them (or its state certifying agency, if applicable) of how it intends to fulfill its obligations with respect to medical records.
Read the article “FDA Offers Inside Look at Mammography Patient and Provider Notification Process.”
For more information: www.fda.gov


 March 06, 2026
March 06, 2026 









