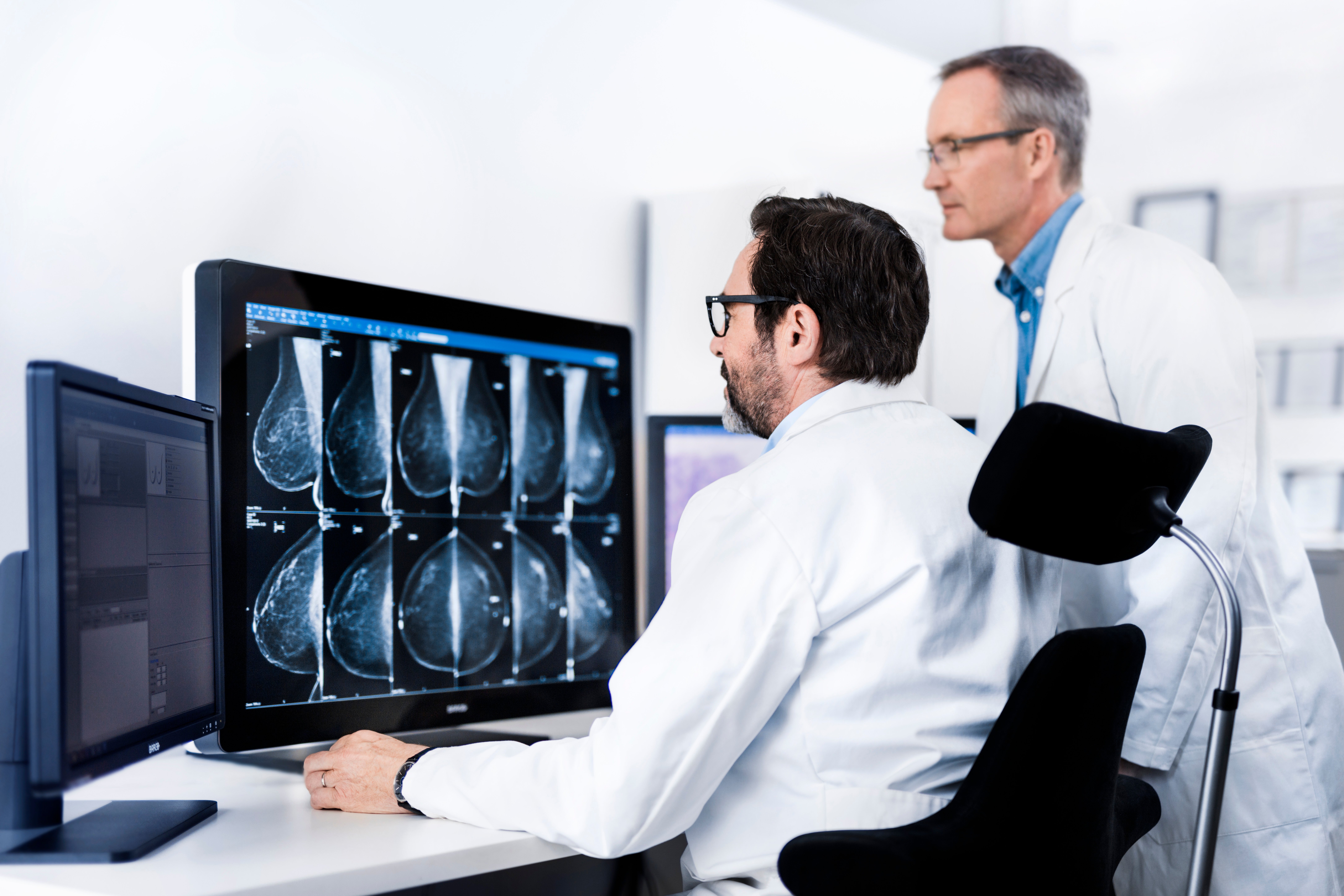
October 16, 2018 — The Mammography Quality Standards Act (MQSA) was enacted to improve the quality of mammography and ensure that all women across the country can count on receiving a mammogram that meets quality standards. The MQSA program has many components, and here the U.S. Food and Drug Administration (FDA) explains how they all fit together:
The MQSA Statute and its implementing Regulations together shape the MQSA program. The program was established through the legislative process, with the passage of a statute (or Act) by Congress, signed into law by President George H.W. Bush in 1992, and then the publication of implementing Regulations by the FDA, later supplemented with explanatory Guidance.
The MQSA Statute, in Title 42, The Public Health and Welfare, of the Code of Federal Regulations (CFR), sets the broad outlines of the requirements. The statute can be found here:
The Act directed the Secretary of the Department of Health and Human Services to promulgate regulations to carry out the Act. The FDA was the agency delegated by the Secretary to do this. The implementing Regulations of the MQSA Statute are in Title 21 of the CFR, part 900, and can be found here:
The Regulations also contain a mechanism for the approval of Alternative Standards when the proposed new standard is determined to be “at least as effective in assuring quality mammography as the standard it proposes to replace.” Different parties, including federal agencies, state governments, accreditation bodies, mammography facilities and equipment manufacturers are qualified to apply for alternatives to various parts of the MQSA standards, respectively, as detailed in in Sec. 900.18 of the Regulations. The 24 currently approved Alternative Standards are listed here:
The MQSA Policy Guidance Help System (PGHS) is a set of informational pages, including citations of regulations, explanatory discussions and tables, and answers to frequently asked questions. They are all intended to help clarify the FDA’s current thinking on various ways to meet the requirements of the regulations. The PGHS contents themselves (except for the citations of regulations) do not have the force of law. Here is a link to the PGHS:
In addition to meeting the MQSA requirements, some aspects of medical practice and radiation protection may also have to meet other state and local requirements, so facility owners, administrators and personnel should make sure they familiarize themselves with all applicable requirements.
There are also clinical practice guidelines for mammography published by various professional organizations. These are not requirements which must be met, but recommendations which may be helpful in maintaining quality practice.
The relationship between the Act, Regulations, PGHS, Alternative Standards and clinical practice guidelines, can best be explained using this example regarding the communication of mammography results:
- The Act (or Statute): In order to promote clear communication of mammography results, the Act requires (among other things) that a certified facility “must assure the preparation of a written report of the results of any mammography examination,” and provide that written report to the patient’s referring physician (if any). The regulations also specify the timeframe for issuing the written report.
- The Regulations: These list specific items that must be included in the mammography report, including (among others) an “[o]verall final assessment of findings, classified in one of [a specified list of] categories.” (Note that MQSA does not regulate what findings make a mammogram Negative, Benign, Suspicious, etc. – it only requires that, after the exam is interpreted, one of these approved assessments must be used in the report, to promote clear communication of the results.)
- The PGHS: This lists acceptable slight variations in the wording of the final assessment categories listed in the Regulations.
- MQSA Alternative Standards: Two of the approved Alternative Standards, No. 11 and No. 12, address the assessment categories in mammography reports. These standards add two new approved assessments: one for cases which require comparison to prior mammograms, and one for cases in which a post-procedure mammogram was performed to assess the placement of a tissue marker. These assessments may be used by any facility but are not required to be used.
- Practice guidelines: These may be issued by various professional societies, academic and health organizations. They may contain recommendations for good practice regarding communication and the contents of mammography reports, but the MQSA regulations do not require that these recommendations be followed.
Thus, the MQSA Act, Regulations (and approved Alternative Standards), and PGHS all help to gain an understanding of the requirements for certified mammography facilities. Practice guidelines may further help to enhance the quality of mammography, though they are not part of the MQSA program.
For more information: www.fda.gov
Related MQSA Content
What It Takes to Remain an MQSA-Qualified Inspector
FDA Article Underscores Benefits of Adequate Breast Compression for Mammography Image Quality


 February 18, 2026
February 18, 2026 









