July 25, 2024 — The radiology gender gap is decreasing, but there remains work to be done, according to an editorial published today in RadioGraphics, a journal of the Radiological Society of North America (RSNA). In 2022, nearly half of residents and fellows in Accreditation Council for Graduate
July 26, 2024 — GE HealthCare and Amazon Web Services, Inc. (AWS), an Amazon.com, Inc. company, announced a strategic ...
July 25, 2024 — Immunis, Inc., a clinical-stage biotech developing groundbreaking secretome therapeutics for age and ...
July 25, 2024 — The radiology gender gap is decreasing, but there remains work to be done, according to an editorial ...
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
July 25, 2024 — NorthStar Medical Radioisotopes, LLC and BWXT Medical Ltd., a subsidiary of BWX Technologies, Inc ...
July 25, 2024 — Positron Corporation, a leading molecular imaging medical device company offering PET & PET-CT imaging ...
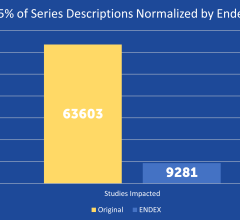
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
July 24, 2024 — Telix Pharmaceuticals Limited announced that the United States (U.S.) Food and Drug Administration (FDA) ...
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
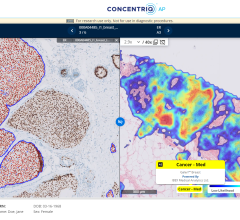
July 24, 2024 — Proscia, a developer of artificial intelligence (AI)-enabled digital pathology solutions for precision ...
July 23, 2024 — Professional registration is open for RSNA 2024, the world’s largest radiology forum. This year’s theme ...
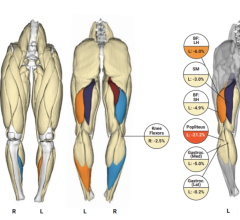
July 23, 2024 — Researchers at the National Institutes of Health (NIH) found that an artificial intelligence (AI) model ...
Having the most efficient clinical workflows with enhanced diagnostic capabilities is a major goal for clinicians and ...
July 23, 2024 — EMVision, an Australian medical device company focused on the development and commercialization of ...
July 22, 2024 — Healthcare artificial intelligence (AI) systems provider, Qure.ai, has announced its receipt of a Class ...
July 22, 2024 — RefleXion Medical, an external-beam theranostic oncology company, today announced that researchers from ...
Clinicians and referring physicians need fast, secure access to both patient data and their diagnostic or viewing tool ...
July 19, 2024 — GE HealthCare announced it has entered into an agreement to acquire Intelligent Ultrasound Group PLC’s ...
July 19, 2024 — Core tactics to address the current medical imaging and radiation therapy workforce shortage and build ...
July 18, 2024 — NeuroLogica Corp, a subsidiary of Samsung Electronics Co. Ltd., announced its latest configuration of ...
July 18, 2024 — The members of the American Society for Radiation Oncology (ASTRO) recently elected five new officers to ...
July 18, 2024 — At the Annual Meeting of AHRA (the Association for Medical Imaging Management), Agfa Radiology Solutions ...
July 17, 2023 — The Radiological Society of North America (RSNA) Research and Education (R&E) Foundation Board of ...
July 17, 2024 — LG Electronics (LG) is accelerating its B2B medical device business, expanding its lineup of diagnostic ...
July 17, 2024 — Hyperfine, a groundbreaking medical device company that has redefined brain imaging with the world’s ...






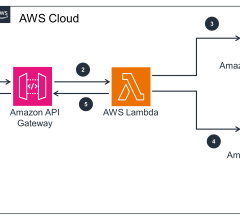
 July 26, 2024
July 26, 2024