Long before U.S. President George W. Bush emphasized the importance of the electronic health record (EHR), cancer ...
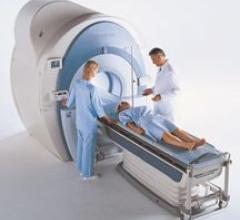
Toshiba’s high-performance Excelart Vantage ZGV magnetic resonance imaging (MRI) system received FDA clearance. The new Vantage ZGV is immediately available and offers new product sequences, enhanced image quality and features the Mach 8 processor, which increases reconstruction to 1,300 images per second for more powerful clinical applications.
Forty-five percent of the rise in Medicare spending between 1999-2003 was attributed to medical imaging. Although the ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
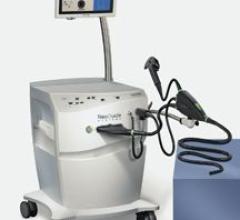
NeoGuide Systems has announced that it received FDA 510(k) clearance to market the NeoGuide Endoscopy System. The system enhances physician control of the colonoscope and eliminates looping, the principle reason why conventional colonoscopy procedures are painful, time consuming and difficult.
Bringing advanced ventilation technology into the neonatal intensive care unit (NICU), Maquet’s SERVO-i Infant Release 3 ...
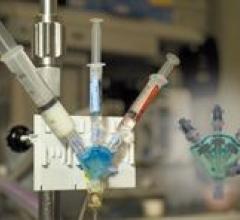
IntraSafe Medical received FDA clearance to market its SureFlow Safety Infusion Device, a multiport access device that ...
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
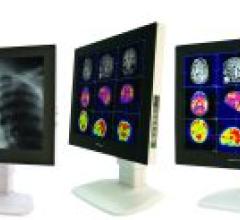
Ampronix will feature its new Modalixx G202MP, GC8 and GC212HB at RSNA 2008. The series feature a complete set of inputs which cover a wide range of video parameters and connectivity options used for MRI, CT, endoscopy, radiographics, DR/CR, cath lab, mobile C-arm, portable X-ray DR/CR, PET and others.


 May 14, 2006
May 14, 2006