June 10, 2008 - At the Digestive Disease Week Conference, Cook Medical announced that it has been granted 510(k) clearance from the FDA for use of the Evolution Controlled Release Esophageal Stent System, a stent designed to improve the quality of life for patients with esophageal cancer.
June 10, 2008 - Wright Medical Group Inc., an orthopedic medical device company, said it has acquired certain assets of ...
Cook Medical's FDA-cleared Evolution Controlled Release Esophageal Stent System is a stent designed to improve the quality of life for patients with esophageal cancer.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
June 10, 2008 – Screening for lung cancer with computed tomography (CT) may help reduce lung cancer deaths in current ...
June 10, 2008 - World-wide inventors and leaders in radio frequency identification (RFID), bar coding, and other ...
Small and large medical imaging equipment suppliers alike are struggling with slowed equipment sales resulting from ...
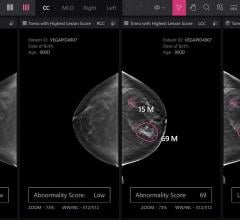
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
June 10, 2008 - The U.S. Department of Health and Human Services (HHS) June 3 released two draft guidance documents for ...
June 9, 2008 - At the request of its membership, the American College of Physicians (ACP) recently conducted an ...
The FDA granted tentative approval for Covidien’s Abbreviated New Drug Application (ANDA) for its Kit for the Preparation of Technetium Tc 99m Sestamibi Injection. Covidien’s tentatively approved product is a generic of Cardiolite, which is a myocardial perfusion imaging agent used for detecting coronary artery disease.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
June 9, 2008 - Siemens unveiled the ACUSON SC2000 volume imaging ultrasound system, which acquires nonstitched, real ...
June 9, 2008 - The administration of imprecise dosages, particularly to small children, remains a serious problem faced ...
The EmpowerMR injector system has multiple features created to cope with the problem of electrical interference in the ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
The FDA recently approved Covidien’s contrast delivery system with radiofrequency identification (RFID) technology ...
With radiologists in short supply, the need for teleradiology services continues to grow and the market has responded in ...
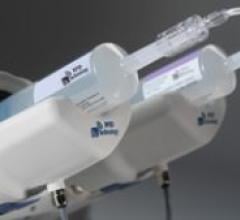
The ACIST CVi Contrast Delivery System provides one injection system for all cardiac and vascular angiography procedures. The variable-rate ACIST CVi system aims to provide ease of operation for clinicians, while obtaining quality images with less contrast used and delivered to the patient.
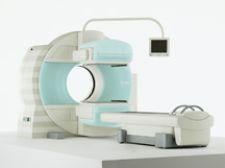
GE received FDA clearance for its new Signa MR750 3.0T scanner that delivers up to 60 percent additional anatomical ...
June 9, 2008 – American TeleCare Inc. (ATI) released Quick Notes as a new feature of its inLife/LifeView Telehealth ...
The Codonics Virtua Medical Disc Publisher is designed to set a new standard for speed, efficiency and ease of use in ...
The FDA cleared GE Healthcare’s new LightSpeed CT750 HD, a high-definition CT scanner that is said to produce images 100 ...
June 9, 2008 - Siemens Medical Solutions gained FDA 510(k) market clearance for the SOMATOM Definition AS, what is said ...

 June 09, 2008
June 09, 2008