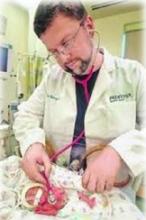
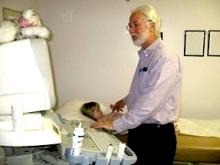
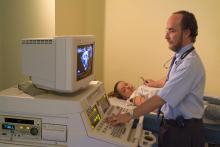
June 13, 2012 — M*Modal Inc. announced the M*Modal Catalyst family of cloud-based applications, enabling healthcare decision-makers to extract actionable information from “unstructured” medical documentation, regardless of how that data was created, and combine it with structured data contained in electronic clinical systems.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now