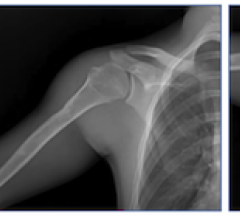
Adaptix Ortho350 (Photo: Adaptix Ltd.)
Nov.10, 2025 — Medical imaging technology company Adaptix Ltd. has received 510(k) clearance from the U.S. Food and Drug Administration for its orthopedic 3D imaging product, the Adaptix Ortho350.
Based in the Oxford University Science Park in the United Kingdom, Adaptix is on a mission to transform radiology by offering affordable, low-dose 3D Digital Tomosynthesis (DT) imaging at the point-of-care.
The new Adaptix Ortho350 is a compact, mobile, low-dose DT orthopedic imaging system capable of delivering fast, lower-cost, 3D X-ray imaging at the point-of-care. Developed specifically to offer 3D X-ray imaging of upper and lower extremities; hands, elbows, knees and feet, at a fraction of the radiation dose of traditional CT systems. The Adaptix Ortho350 system provides clinicians with clearer images than 2D X-ray systems, offering advantages in terms of fewer acquisitions, accelerated patient workflow and enhanced diagnostic accuracy.
Adaptix is already serving veterinary and industrial markets using its revolutionary technology in the form of the Adaptix VetSA3D veterinary imaging product and the Adaptix NDT3D industrial Non-Destructive Test products. Receiving the FDA 510(k) clearance will open up the human imaging market for its technology in the US and allow Adaptix to deliver on its mission to transform radiology through provision of innovative 3D imaging technologies into healthcare providers.
Sarah Small, Adaptix’s CEO, stated, “Securing this FDA 510(k) clearance represents a significant milestone for Adaptix in our mission to transform radiology. We already have a great deal of interest from healthcare providers and clinicians for our revolutionary orthopedic imaging system. We are looking forward to delivering “3D-First” enhanced orthopedic DT imaging across Primary Care, Intensive Care and Emergency Departments throughout the US and beyond.”
More information is available at www.adaptix.com.


 March 06, 2026
March 06, 2026