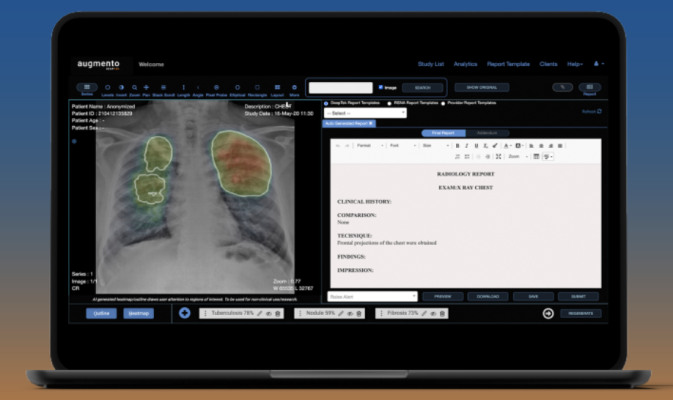
Medical imaging AI company DeepTek.ai is showcasing its US FDA-cleared chest X-ray reporting AI solution, Augmento X-Ray, during HIMSS 2024 being held March 11-15 in Orlando, FL. Image courtesy: DeepTek.ai
March 8, 2024 — DeepTek.ai, a leading medical imaging AI company, will showcase its groundbreaking US FDA-cleared chest X-ray reporting AI solution, Augmento X-Ray, at the upcoming Health Information Management Systems Society conference, HIMSS 2024, taking place March 12th-14th, 2024, at booth #4661.
HIMSS (Healthcare Information and Management Systems Society) is the world's largest health information and technology (Health IT) conference, attracting tens of thousands of healthcare professionals, industry leaders, and innovators from around the globe.
Augmento X Ray is a pioneering CADe (Computer-Aided Detection) chest X-ray AI solution designed to redefine X-ray reporting and address the growing global burden of chest X-ray interpretation. With over 1.5 billion chest X-rays conducted annually, radiologists face immense pressure to deliver an accurate and timely diagnosis.
This advanced AI solution leverages deep learning algorithms to analyze chest X-rays and automatically identify, categorize, and highlight suspicious areas. Unlike traditional point AI solutions focused on specific pathologies, Augmento X-Ray offers a comprehensive analysis, covering a broad range of lung, pleural, cardiac pathologies, and foreign bodies/hardware, making it versatile and adaptable to various clinical scenarios.
DeepTek’s AI solutions have been transforming healthcare in India and the Asia-Pacific region by providing increased access to quality care at a lower cost. Over 500 hospitals and imaging centers are using DeepTek AI solutions, impacting the lives of 100,000 people monthly. In Singapore, DeepTek's US FDA cleared platform, Augmento, has been deployed as the National Radiology AI platform, boosting productivity and quality of care across all public hospitals. With its operations in Pune; DeepTek has about 200 members and partners like TATA Capital, NTT DATA Japan, and Shimadzu Asia Pacific.
DeepTek.ai is committed to advancing the field of radiology through cutting-edge AI technology. Visit booth #4661 at HIMSS 2024 to experience live demonstrations of the Augmento platform and learn how DeepTek.ai is shaping the future of medical imaging.
For more information: www.deeptek.ai


 February 06, 2026
February 06, 2026 









