June 14, 2023 —Avenda Health, an AI healthcare company creating the future of personalized prostate cancer care, today announced the results of a new study, “Prediction and Visualization of Lesion Extent in Intermediate-Risk Prostate Cancer using Artificial Intelligence,” published in the European Urology Open Science. Led by Stanford University School of Medicine’s Department of Urology, researchers found that Avenda Health’s machine learning (ML) model, Unfold AI, and novel cancer probability metric was shown to be accurate and effective in identifying the extent of the cancer, not just location. The study demonstrates considerable promise for the technology and warrants further study of the treatment platform.
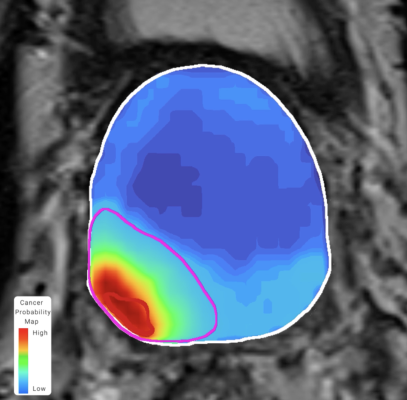
The study indicates that physicians armed with Unfold AI can better determine the ideal course of treatment, including surgery, focal therapy and active surveillance, compared to standard imaging like MRI. The 3D cancer visualization ensures physicians are removing or monitoring the entirety of the cancer, significantly reducing the risk of leaving cancer behind or causing damage to sensitive urinary and sexual tissue. Using Unfold AI, physicians can support the patient throughout their entire cancer journey, from screening patients before treatment to determine the location of the cancer as well as rescreening post-treatment to inform next steps.
The study, including 50 prostate cancer patients, found that the ML-driven approach that incorporates both biopsy and image features would be better equipped to define cancer risk and treatment margins than the current standard of care. The ML model was shown to be an effective tool for defining cancer margins. In particular, the rate of complete clinically significant prostate cancerencapsulation (80% versus 56%) for ML model margins dwarfed that of hemi-gland margins (i.e. full-gland removal). Clinically significant disease occurred beyond a single hemisphere for nearly half of cases, strongly suggesting that hemisphere-based margin boundaries should be avoided in favor of a more patient-specific treatment planning approach, including surgery, focal therapy and active surveillance.
“After nearly a decade of research and development, we are thrilled to see these promising results from the dedicated team at Stanford University,” said Shyam Natarajan, Ph.D., co-founder and CEO of Avenda Health. “The prostate cancer space has seen little to no advancement in nearly 40 years, and we look forward to bringing this important information to more prostate cancer patients in the coming year.”
“Our mission at Avenda Health has always been to bring personalized care to prostate cancer patients to improve quality of life. These study results will be instrumental in forging a path forward in providing an alternative to the standard of care and giving urologists and their patients the confidence in providing an effective prostate cancer treatment plan,” said Brittany Berry-Pusey, Ph.D. co-founder and COO of Avenda Health.
One in eight men will develop prostate cancer in their lifetime. The current one-size-fits-all approach of treating the entire prostate‒because current MRI technology cannot identify the full extent of the tumor and cancer growth within the organ‒results in nearly 50% of patients losing their sexual or urinary function. Avenda Health’s ML-driven approach personalizes the patient’s treatment journey providing options to preserve quality of life and prevent recurrence. Providing physicians with a personalized, 3D visualization of the cancer offers a better informed decision on the course of treatment. Avenda Health’s prostate cancer management platform, Unfold AI, received FDA clearance in late 2022, opening the door for more precise prostate cancer treatments using AI.
For more information: avendahealth.co


 February 06, 2026
February 06, 2026 









