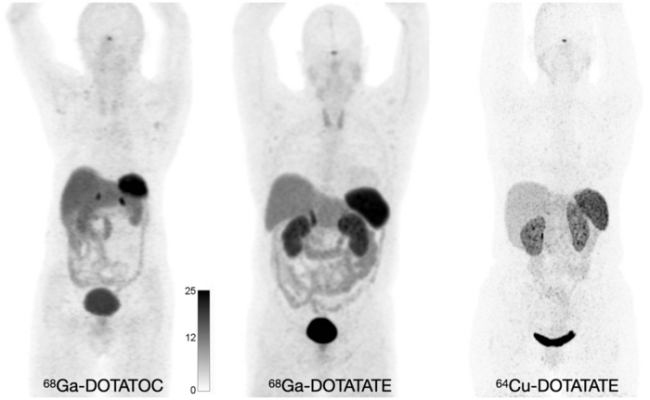
Normal biodistribution of 68Ga-DOTATOC, 68Ga-DOTATATE, and 64Cu-DOTATATE. Image courtesy of Hope TA, Allen-Auerbach M, Bodei L, Calais J, et al., SNMMI Procedure Standard/EANM Practice Guideline for SSTR PET: Imaging Neuroendocrine Tumors. J Nucl Med. 2023 Feb;64(2):204-210.
February 23, 2023 — The Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the European Association of Nuclear Medicine (EANM) have issued a new procedure standard/practice guideline for somatostatin receptor (SSTR) PET imaging for patients with neuroendocrine tumors (NETs). The standard/guideline, published in the February issue of The Journal of Nuclear Medicine (JNM), is intended to assist physicians in recommending, performing, interpreting, and reporting the results of SSTR PET imaging studies for patients with NETs.
SSTRs are overexpressed on a wide range of NET cells and can be targeted using somatostatin analogs (SSAs). While SSAs were initially targeted with 111In-pentetreotide scintigraphic imaging, the next generation of SSTR PET imaging with 68Ga-DOTATATE, 68Ga-DOTATOC, or 64Cu-DOTATATE, allows for improved sensitivity of lesion detection, lower radiation dose, and shorter and more convenient study duration. The new standard/guideline focuses on these PET radiotracers.
In the SSTR PET imaging standard/guideline, SNMMI and EANM address common clinical indications, qualifications and responsibilities of personnel, procedure/specifications of the examination, documentation and reporting, and dosimetry. They also present standardized quality control/quality assurance procedures and imaging procedures for SSTR PET, as adequate precision, accuracy, repeatability, and reproducibility are essential for the clinical management of patients and the use of SSTR PET within multicenter trials.
“The SSTR PET procedure standard/practice guideline aims to provide clinicians with the best available evidence, to inform where robust evidence is lacking, and to help them to deliver the best possible diagnostic efficacy and study quality for their patients,” said Thomas A. Hope, MD, vice chair of Clinical Operations and Strategy in the Department of Radiology and director of Molecular Therapy at the University of California San Francisco in San Francisco, Calif., and chief of Nuclear Medicine at the San Francisco VA Medical Center. “A standardized imaging procedure will help to promote the appropriate use of SSTR PET and enhance subsequent research.”
SNMMI/EANM periodically define new standards/guidelines for nuclear medicine practice to help advance the science of nuclear medicine and to deliver effective and safe medical care to patients. Each standard/guideline, representing a policy statement by the SNMMI/EANM, undergoes a thorough consensus process in which it is subjected to extensive review.
For more information: www.snmmi.org


 February 13, 2026
February 13, 2026 









