April 8, 2022 — Toronto-based Molli Surgical is gaining momentum, providing its patient-first solution to University Health Network’s (UHN) Sprott Department of Surgery. Molli Surgical’s Canadian-designed, wire-free and radiation-free marker technology for localizing lesions for breast cancer surgery will improve patient and physician comfort and confidence.
“We are gratified and honored to work with the UHN — a world leader in improving patient access to quality care,” said Fazila Seker, PhD, President and CEO of Molli Surgical Inc. “The Princess Margaret Cancer Centre at UHN is the latest hospital to adopt Molli to provide a better experience for patients, increase ease and capacity for surgical and radiology departments, and simplify the breast cancer treatment experience.”
UHN is world-renowned for their industry leading scientific research and focus on excellence in patient care and safety. They are committed to innovations like wire-free localization that improve the perioperative journey for all breast surgery patients. Going forward, Molli Surgical will be the provider of wire-free, radiation-free technology used in lumpectomy and mastectomy procedures.
“Localizing breast lesions with wires happened on the same day as a patient’s surgery. This can be challenging, since it involves going through a procedure right before their operation, when patients are already fasting and may be anxious,” said Dr. Tulin Cil, Breast Surgical Oncologist and Gattuso Chair in Breast Surgical Oncology, UHN.
“With Molli, we have different options for scheduling the timing of that procedure. Now, patients can go home comfortably between localization and their surgery. I find it is easy to use, efficient and precise. It allows us to localize the tissue accurately and safely with an improved overall patient experience.”
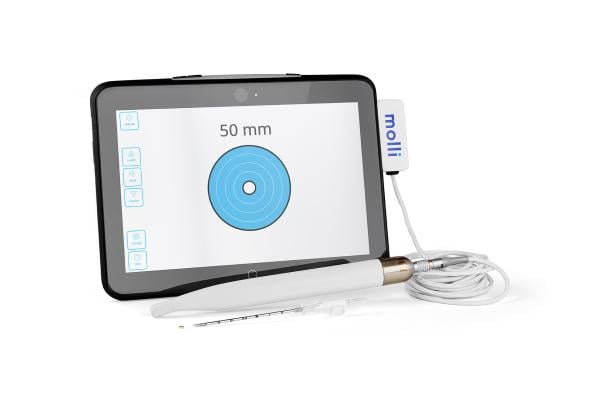
Molli Surgical’s Health Canada approved and FDA-cleared Molli features a 3.2 mm marker designed to put the breast cancer patient first by providing a better experience over traditional wire and other localization options. The Molli Wand detects the Molli Marker and visualizes its location on a tablet — much like a GPS — helping surgeons locate lesions more efficiently and with improved accuracy, which translates into better cosmetic outcomes for patients. The marker is implanted by a radiologist within 30 days of surgery. By decoupling localization of the lesion from removal, Molli allows for more efficient clinical workflows in radiology and surgery departments, while allowing for greater comfort and convenience for patients. This increased efficiency leads to increased productivity for hospitals.
“Molli is an elegant solution on a number of different fronts: with its scheduling flexibility, it helps to improve clinical workflow. It comes with a reasonable cost, and a team that’s simply a pleasure to work with. Most importantly, with its less-invasive nature, patients see a reduction in pain and anxiety,” said Terri Stuart-McEwan, Executive Director - University Health Network Sprott Department of Surgery. “This is an important step towards reaching our strategic goals.
The partnership between Molli Surgical and the Princess Margaret will provide us with support and expertise as we move forward with improving patient care.”
Molli is also a solution for hospitals using radioactive technology, which requires extensive safety regulations surrounding radiation and disposal protocols. Molli’s ease-of-use allows for quick adoption by doctors and its robust, intuitive design facilitates smooth adoption by care providers and hospital systems.
For more information: http://mollisurgical.com/


 February 06, 2026
February 06, 2026 









