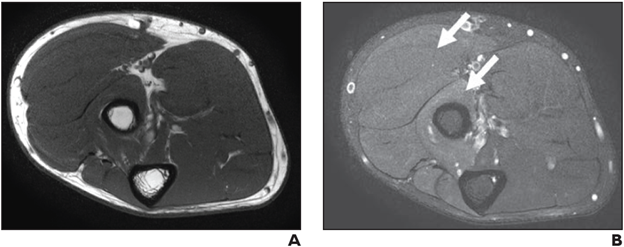
Neuropathy Score Reporting and Data System (NS-RADS) Subclass M1 (Edemalike Signal): Axial T1-weighted (A) and STIR (B) images depict denervation edemalike signal of extensor muscles of dorsal and mobile wad compartments with no fatty infiltration or atrophy consistent with NS-RADS M1.
March 4, 2022 — According to an article in ARRS’ American Journal of Roentgenology (AJR), a new reporting and data system for standardized evaluation and recording of peripheral nerve pathologies—Neuropathy Score Reporting and Data System (NS-RADS)—has been created and validated in a multicenter study.
“The proposed NS-RADS classification is accurate and reliable across different reader experience levels and a spectrum of peripheral neuropathy conditions,” wrote lead researcher Avneesh Chhabra of UT Southwestern in Dallas, TX. “NS-RADS can be used as a standardized guideline for reporting peripheral neuropathies and improved multidisciplinary communications.”
Chhabra and team’s retrospective study included 100 patients with nerve imaging examinations and known clinical diagnoses. Utilizing mutually agreed-upon qualitative benchmarks for classifying and grading peripheral neuropathies, different classes were established to account for the spectrum of underlying pathologies (unremarkable, injury, neoplasia, entrapment, diffuse neuropathy, not otherwise specified, and postintervention state) with subclasses to describe lesion severity or extent. Validation was performed by 11 fellowship-trained musculoskeletal radiologists across 10 institutions, and after initial multimedia training, all 100 cases were blind-presented to readers.
Offering a uniform lexicon and practical guideline for reporting neuropathic conditions on MRI, ultimately, NS-RADS accuracy for determining milder versus more severe categories per radiologist ranged from 88% to 97% for nerve lesions and from 86% to 94% for muscle abnormalities.
“On the basis of the overall promising interrater agreement shown in this study, we believe that the newly proposed NS-RADS classification will perform as well in routine practice as it did in this initial validation study,” the authors of this AJR article concluded.
For more information: arrs.org


 February 13, 2026
February 13, 2026 









