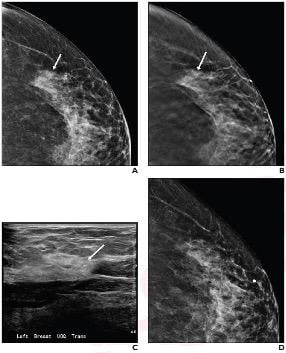
57-Year-Old Woman Presented for Screening Mammography: Digital, A, and tomosynthesis, B, craniocaudal images showed 1.8 cm asymmetry (arrows) lateral left breast categorized as BI-RADS 0. C, Ultrasound left breast evaluation revealed normal fibroglandular tissue (arrow) believed to correlate with mammographic finding. D, Asymmetry effaced on rolled lateral craniocaudal imaging. Metallic BB marker noted at 12 o’clock position, anterior depth. Final assessment BI-RADS 1. Image courtesy of ARRS
January 17, 2022 — According to an article in ARRS’ American Journal of Roentgenology (AJR), radiologists should consider performing ultrasound (US) first for digital breast tomosynthesis (DBT)-recalled noncalcified masses.
“Omitting diagnostic mammography when US is negative has a low false-negative rate,” wrote lead researcher Jessica H. Porembka, M.D., from the University of Texas Southwestern Medical Center in Dallas. Noting that US alone is effective in diagnosing noncalcified masses recalled on screening tomosynthesis, “for asymmetries,” she explained further, “diagnostic mammography may be best without the need for additional US, while architectural distortions still warrant diagnostic mammography and US.”
Per the protocol of Porembka et. al’s HIPAA-compliant, IRB–approved prospective study, 399 women (mean age, 60 years) recalled for noncalcified lesions from screening DBT underwent initial diagnostic US from July 2017 to June 2019. Imaging decisions—determined via BI-RADS assessments and management recommendations, biopsy outcomes, and follow-up—were recorded using case reports filed on the day of the diagnostic evaluation.
Ultrasound alone without mammography adequately completed the diagnostic evaluation of 71.2% (306/430) of noncalcified lesions recalled from screening tomosynthesis, 93.7% (178/190) of recalled masses, and diagnosed 92.1% (35/38) of cancers, yielding a sensitivity of 94.9% for cancer diagnosis.
“A large number of screening tomosynthesis–detected noncalcified masses may be further evaluated with ultrasound alone, without compromising diagnostic sensitivity,” the authors of this AJR article reiterated.
For more information: www.arrs.org


 February 18, 2026
February 18, 2026 









