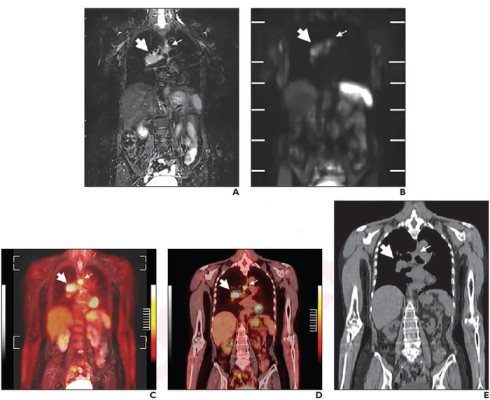
Per reference-standard diagnosis, TNM stage was IIIA (T3N2M0) and Veterans Administration Lung Cancer Study Group stage was LS. Coronal (A) STIR image and (B) DWI from whole-body MRI. Primary lesion in right hilum (thick arrow) was suspected to be invading right main bronchus, thus assessed as T3. Ipsilateral mediastinal lymph node (thin arrow) was suspected to be metastatic, and N category was thus assessed as N2. (C) Coronal fused PET and STIR image from coregistered FDG PET/MRI show primary lesion in right hilum (thick arrow) and mediastinal lymph node metastasis (thin arrow). (D) Coronal fused image from integrated FDG PET/CT shows primary lesion in right hilum (thick arrow) and mediastinal lymph node metastasis (thin arrow). (E) Coronal image from unenhanced CT shows primary lesion in right hilum (thick arrow) and mediastinal lymph node metastasis (thin arrow). Primary lesion was not suspected to invade right main bronchus invasion. Thus, whole-body MRI, FDG PET/MRI, and FDG PET/CT accurately assessed patient as stage IIIA (T3N2M0) and LS disease. Conventional imaging accurately assessed LS disease, but incorrectly assessed patient as stage IIIA (T2bN2M0). Image courtesy of American Roentgen Ray Society (ARRS), American Journal of Roentgenology (AJR)
December 10, 2021 — According to an article in ARRS’ American Journal of Roentgenology (AJR), MRI—with or without FDG PET coregistration—can improve the staging of patients with small cell lung cancer (SCLC).
“FDG PET/CT, whole-body MRI, and coregistered FDG PET/MRI outperformed conventional tests for various staging endpoints in patients with SCLC,” concluded first author Yoshiharu Ohno from the Fujita Health University School of Medicine in Japan. Whole-body MRI and FDG PET/MRI outperformed FDG PET/CT for T category and thus TNM stage, “indicating utility of MRI for assessing extent of local invasion in SCLC.”
Ohno and colleagues’ prospective study included 98 patients (64 men, 34 women; median age, 74 years) with SCLC who underwent conventional staging tests (brain MRI; neck, chest, and abdominopelvic CT; bone scintigraphy), FDG PET/CT, and FDG PET/MRI within 2 weeks before treatment. After MRI technologists performed coregistration via proprietary software (Canon Medical Systems), two nuclear medicine physicians and two chest radiologists independently reviewed the examinations in separate sessions.
In patients with SCLC, accuracy for T category was higher (p<.05) for whole-body MRI (94.9%) and FDG PET/MRI (94.9%) than for FDG PET/CT (85.7%). Meanwhile, TNM stage accuracy was higher (p<.05) for whole-body MRI (88.8%) and FDG PET/MRI (86.7%) than for FDG PET/CT (77.6%) and conventional staging tests (72.4%).
“These additional observations may relate to a superior role of MRI in assessing the extent of local soft tissue invasion by tumor, as has been observed in settings other than SCLC,” added the authors of this AJR article.
An electronic supplement to this AJR article is available here.


 February 13, 2026
February 13, 2026 









