Lunit, a leading medical AI provider, announced that the U.S. Food and Drug Administration (FDA) 510(k) has cleared its AI solution for breast cancer detection, Lunit INSIGHT MMG. Along with its chest X-ray triaging solution, Lunit INSIGHT CXR Triage, the company’s AI solution for both chest X-ray and mammography is now commercially available across the U.S.
“I am delighted to deliver the great news and to introduce Lunit INSIGHT MMG to healthcare professionals and institutions across the US,” said Brandon Suh, CEO of Lunit. “With our AI solution, we hope to increase the efficiency and accuracy of mammography screening as well as chest x-ray triaging. We can assist radiologists diagnose diseases at an earlier stage, helping patients be treated at the right time.”
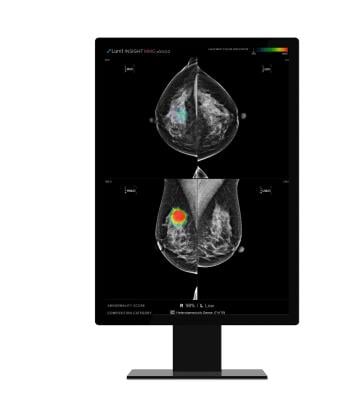
Lunit INSIGHT MMG is one of the company’s most mature radiology products which analyzes mammography images with high accuracy. It provides the location of lesions suspicious of breast cancer and an abnormality score that reflects the AI’s confidence of the existence of detected lesions.
The product is trained with a large-scale data of more than 240,000 mammography cases that include up to 50,000 breast cancer cases and is known to show excellent performance in finding breast cancer at an earlier stage.
According to a study published in JAMA Oncology in 2020, Lunit INSIGHT MMG showed the best accuracy among three commercialized AI to identify breast cancer. It was found to have around 15% higher sensitivity compared to the other two algorithms. The FDA-cleared AI solution has been previously CE marked and approved for commercial sales in more than 35 countries worldwide.
“This is a huge milestone and business opportunity for us, as the U.S. is one of the largest and most important markets that covers more than 45% of the global breast imaging market,” added Suh. “Based on the FDA clearance, we will not only bring diagnostic value to medical professionals and patients in the U.S. but leverage our business to be a leading global provider of breast AI solution.”
Lunit INSIGHT MMG will be showcased at RSNA 2021, AI Showcase - booth #4545, Nov. 28 – Dec. 2, 2021, in Chicago.
For more information: https://www.lunit.io/en


 February 11, 2026
February 11, 2026 









