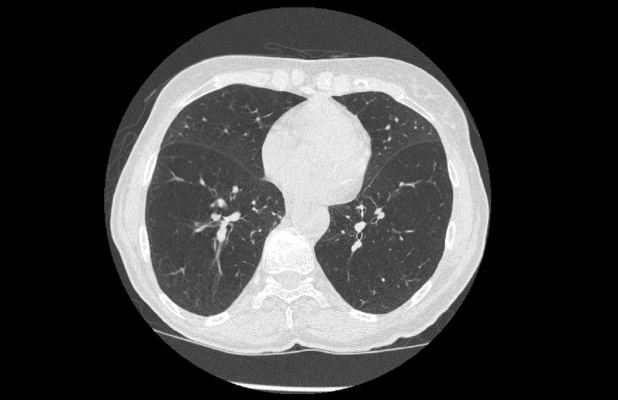
November 23, 2021 — Median Technologies announces new outstanding performance of its lung cancer screening (LCS) CADx1 to characterize malignant vs. benign lung nodules. New results specifically focus on the characterization of stage 1 lung cancer nodules, using Median's proprietary iBiopsy deep learning algorithm on low-dose chest computed tomography (LDCT).
Lung cancer is the number one cancer killer globally, causing an estimate of 1.8m deaths, accounting for 18% of all cancer deaths in 2020. This is largely due to late-stage diagnosis of the disease associated with poor five-year survival. Based on information from the International Association for the Study of Lung Cancer (IASLC) staging project, the five-year overall survival rate for patients with stage 1 non-small cell lung (NSCLC) cancer is 68-92% whereas it is only 1-10% for patients with stage 4, and unfortunately over 40% of patients already have stage 4 disease at the diagnosis without screening. A noteworthy International Early Lung Cancer Action Program (I-ELCAP) cohort study of stage 1 cancer patients revealed an excellent 15-year LCS survival of 92% which again serves to show how critical early-stage diagnosis via lung cancer screening remains. Thus, discriminating between benign nodules and stage 1 cancers is crucial to catch the cancer when it is still manageable and curable, which is key in saving patients' lives.
New results released today are based on a cohort of 1,737 patients from the National Lung Screening Trial cases (NLST) consisting of a total of 16,249 lung nodules. The training set included 1,239 patients with 11,676 nodules and the test set included 498 patients having a total of 4,573 nodules.
Test sensitivity is the ability of a test to correctly identify patients who actually have the disease: the higher the sensitivity of the test, the lower the percentage of false negatives. Test specificity is the ability of a test to correctly identify patients without the disease: the higher the specificity of the test, the lower the percentage of false positives. In a sub-analysis on the stage 1 lung cancers, results show an AUC of 0.984 with a sensitivity of 93.1 % and a specificity of 96.2%, including stage 1A and 1B lung cancer nodules. For the earliest stage 1A cancer stage nodules, the AUC is 0.982 with a sensitivity of 92.1% and a specificity of 96.2%. Knowing the literature reported over 70% false positive rate with LDCT, Median's offering is poised to help radiologists optimize the management of screening patients helping eliminate the barriers to widespread screening adoption by decreasing false positive rates, and unnecessary biopsies and follow up scans which add to patient stress.
"We are proud of our ability to demonstrate accuracy in the earliest onset of the cancer signals as we've been able to get an astounding 92.1% sensitivity in stage 1A lung cancer at a specificity of 92,6%. This represents a critical shift towards improvement in the patient management and survival, as iBiopsy responds to the problem of false positives, one of the major barriers to the implementation of lung cancer screening programs", said Fredrik Brag, CEO and founder of Median. "We will be presenting our results at the RSNA annual meeting next Nov. 28 during a specific session on AI in medical imaging and we are thrilled to share them with the radiologist community attending the largest imaging conference in the world," he added.
Results will be presented during the AI Showcase Theater at the RSNA Annual Meeting, on Sunday, 28 November, at 12 p.m. CT. Median's team will be available at booth #4849 Level 3 South Hall (AI Showcase) for the duration of the industrial exhibition (Nov. 28 -Dec. 1).
For more information: www.mediantechnologies.com


 March 10, 2026
March 10, 2026 









