January 18, 2021 — AI Metrics, LLC, a medical imaging startup focused on augmented intelligence to improve patient care, announced today that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the company’s flagship image analysis platform.
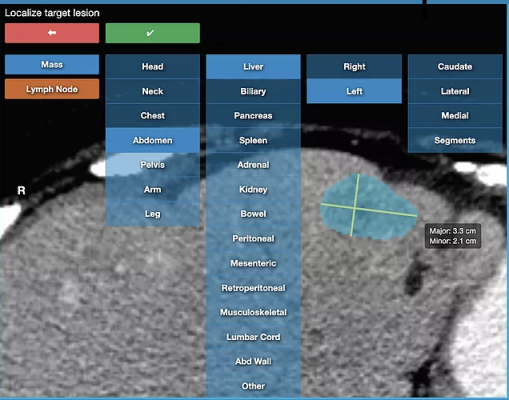
The first application on the platform, AI Mass, assists radiologists with image analysis and reporting of advanced cancer over time. The Company’s innovative approach enables physicians to analyze CT and MRI scans in half the time of traditional methods. AI Metrics has successfully implemented augmented intelligence - the concept of using artificial intelligence (AI) to improve human performance. The AI Metrics platform utilizes AI-assisted workflows to improve accuracy and consistency, and dramatically reduce errors.
“Our goal was to design a simple, intuitive platform in which radiologists could assess medical images from patients with minimal errors and a high level of accuracy, consistency, and efficiency,” said Andrew Smith, M.D., CEO and Founder of AI Metrics. “To do this, we had to break free from outdated methods and create an entirely new image viewer that supports AI and best-practice workflows that generate crystal clear data and reports. We started with advanced cancer and lymphoma, and are now broadening our platform to include early-stage cancers.”
During clinical trials for novel therapeutics, a patient’s response to therapy is assessed over time using detailed criteria. Such criteria require highly-controlled tumor measurements and calculations of percent changes in tumor size. In clinical practice, assessments are more subjective and lack standardization due to time constraints placed upon physicians. “We solved the efficiency and standardization with a combination of AI and guided workflows that capture the best aspects of clinical trials and bring them into clinical practice.”
“This advanced technology completely changes the standard of care - for radiologists, oncologists and patients. As a practicing radiologist and researcher, I’ve envisioned for some time being able to use a system like this, and I am excited to see years of design work finally materialize into an incredible product.” Smith added.
For more information: www.aimetrics.com


 February 16, 2026
February 16, 2026 









