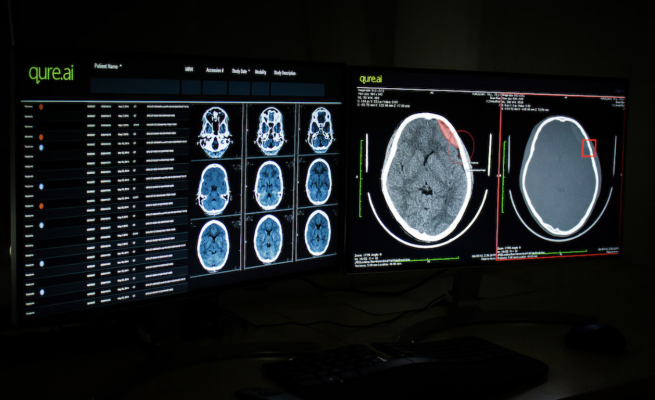
June 30, 2020 — Imaging Artificial Intelligence (AI) provider Qure.ai announced its first US FDA 510(k) clearance for its head CT scan product qER. The US Food and Drug Administration's decision covers four critical abnormalities identified by Qure.ai's emergency room product. Now, the AI tool can be used to triage radiology scans with intracranial bleeds, mass effect, midline shift and cranial fractures. Two of these capabilities — cranial fractures and midline shift— are exclusive to Qure.ai's product. This means that the newly cleared qER suite will be able to triage nearly all critical abnormalities visible on routine head computed tomography (CT) scans.
Every year, over 75 million CT scans are performed in the US, and more than 10,000 people die within 7 days of an emergency room discharge. There is, therefore, a clear need for a comprehensive and high-quality imaging AI tool to assist healthcare providers with prioritization and image interpretation.
"Our target turnaround time for emergent studies is 30 minutes but leveraging AI for acutely critical conditions enables us to shorten that time. For conditions such as intracranial hemorrhage, time is of the essence and those precious minutes can be life-changing for our patients. We have done extensive validation of the Qure.ai qER solution and are excited to continue to partner with Qure.ai and improve care for our patients," said Benjamin W. Strong, M.D., Chief Medical Officer of vRad, the leading teleradiology services provider in the United States.
"Patient outcomes depend directly on the onset-to-treatment time, especially for brain injuries. Every day doctors are required to weigh the benefits of a potentially life-saving surgery versus the risks of an intracranial bleed or other complication. The sooner they have in-depth information that helps them make that decision, the better for the patient. This is where qER plays a key role. We wanted to offer a comprehensive solution to the clinicians, rather than a partial one that triages on the basis of limited or even single findings," said Pooja Rao, Co-founder and R&D head, Qure.ai.
The qER suite plugs directly into the radiology workflow and prioritizes critical cases on the worklist. This triage drastically reduces the time taken to open critical scans, so those with time-sensitive abnormalities get to be read and reported faster, leading to better patient outcomes. The qER product has undergone extensive validation including a 2018 peer-reviewed publication in The Lancet and has been actively deployed at many hospitals and teleradiology providers globally.
Qure.ai's other products include a CE-marked chest X-ray AI tool qXR, and COVID-19 progression monitoring solutions for chest X-rays, with both in clinical use in over 20 countries.
For more information: www.qure.ai


 February 16, 2026
February 16, 2026 









