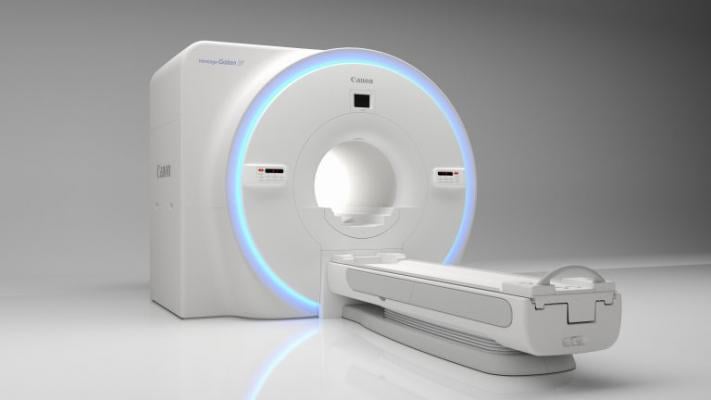
June 15, 2020 — Hospitals and institutions are continually looking for ways to improve diagnostic imaging throughput, especially in today’s environment where disinfection of systems and rooms in between patients is crucial. Now, with the newly FDA-cleared Compressed SPEEDER technology for Canon Medical Systems USA, Inc.’s Vantage Orian 1.5T MR system, clinicians can speed up magnetic resonance imaging (MRI) scan times by reconstructing full resolution images from under-sampled data through iterative reconstruction, improving throughput and allowing for substantial time in between exams to help clean or disinfect the scanner if necessary.
Scan times for MRIs have historically been a challenge. To reduce acquisition times, Compressed SPEEDER, also available on the Vantage Galan 3T MR system, supports image acceleration and can be used to avoid unfolding error artifacts sometimes seen with standard parallel imaging, or can achieve higher resolution in 2-D Fast Spin Echo (FSE) acquisitions. Reduced scan times also enhance patient comfort, which in turn may enable higher quality images by mitigating patient movement caused by patient discomfort during long scans.
Compressed SPEEDER is included in an all-new new version of M-Power software available for the Vantage Orian 1.5T, which also comes standard with Windows 10 and embedded cybersecurity solutions from Canon Medical. Patient data is kept secure with continuous patches and updates from Microsoft, as well as whitelisting functions, which give clinicians access only to applications and processes which have been authorized.
“In MR imaging, shortening scan times is vastly important for both the patient and physician. At Canon Medical, our priority is to support our customers and their patients by providing them with timely advancements that can impact their outcomes and workflow,” said Jonathan Furuyama, managing director, MR Business Unit, Canon Medical Systems USA, Inc. “With the help of Compressed SPEEDER along with Windows 10 on the Vantage Orian 1.5T MR system, healthcare providers can offer a quick, comfortable and safe experience for their patients.”
Foe more information: https://us.medical.canon


 February 16, 2026
February 16, 2026 









