May 21, 2020 — Hologic, Inc. announced that Unifi EQUIP, an automated solution that facilitates compliance with the FDA’s Enhancing Quality Using the Inspection Program (EQUIP) guidance, was recognized as "Best Overall MedTech Solution" in the 2020 MedTech Breakthrough Awards program.
MedTech Breakthrough is an independent organization that recognizes top companies and solutions in the global health and medical technology market. The awards program serves to find and recognize leaders in the most competitive categories of technology.
“We are thrilled to be recognized by MedTech Breakthrough in the Best Overall MedTech Solution category as it underscores our commitment to providing our customers and their patients with an integrated range of best-in-class solutions across the breast cancer continuum of care,” said Pete Valenti, Hologic’s Division President, Breast and Skeletal Health Solutions. “Unifi EQUIP continues to make a positive impact on our customers’ quality assurance efforts and is a key component in our mission to help healthcare providers deliver superior outcomes, at a lower cost, while delivering higher patient satisfaction.”
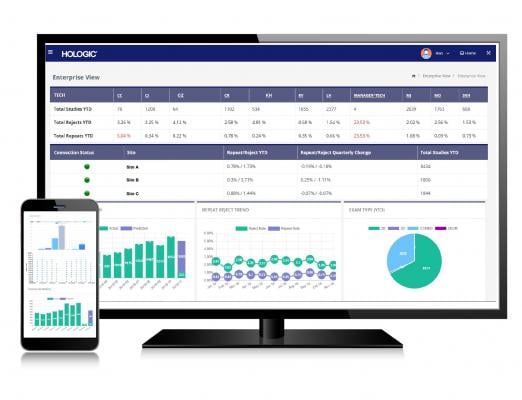
The Unifi Enterprise platform is a comprehensive suite of software solutions that leverages data analytics to optimize the performance of capital equipment and reduce interruptions to patient care by laying a foundation for continued image quality improvement and corrective actions. Unifi Analytics is at the core of the platform, serving as a business intelligence tool for healthcare facilities that allows them to manage their mammography devices, monitor technologist performance, and prevent unanticipated downtime via a predictive tube replacement algorithm.
“Since integrating Unifi EQUIP, our technologists and radiologists have experienced an increase in efficiency in managing, tracking and documenting our clients’ clinical images,” said Denise Hodges, Imaging Center Coordinator, Self Regional Healthcare. “In today’s healthcare landscape, facilities and clinicians are pressured to deliver a high quality of care while identifying cost savings and efficiencies. The entire Unifi Enterprise platform represents a comprehensive solution that allows us to operate efficiently while delivering the best possible care.”
In 2019, Hologic launched Unifi EQUIP in partnership with MagView, a leading mammography information solutions provider. MagView offers solutions for breast imaging reporting, tracking, workflow and compliance, and is utilized by more than 2,500 facilities across the U.S., including many of the nation’s top cancer centers.
For more information: www.hologic.com


 February 05, 2026
February 05, 2026