May 7, 2020 — As COVID-19 makes its way across the globe, our customers are urgently connecting with us to share cases, ideas, and needs. TeraRecon responded by diverting the majority of our engineering and clinical resources to develop and release two innovative and practical solutions in just over three weeks. TeraRecon designed the solutions to address the urgent crisis as well as a much broader view of our customers’ needs—to deliver lasting value as an integrated part of any customers’ lung program or enterprise artificial intelligence (AI) roll-out. We are committed helping our customers leverage technology to extend the reach and responsiveness of care.
These solutions, the Lung Density Analysis II (LDA-II) workflow for Intuition and the Emergency Lung AI Suite, provide two deployment options for rapid access to lung segmentation and quantification tools that can be applied to a wide range of lung illnesses and have been optimized to adapt to the latest disease presentation states.
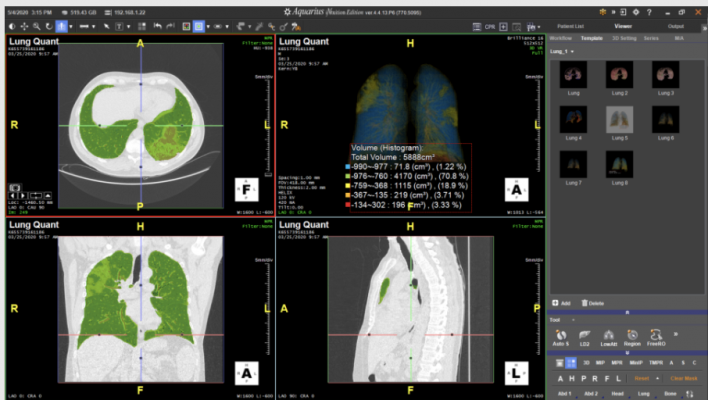
Lung Density Analysis II (LDA-II) for Intuition
The newly released LDA-II allows TeraRecon Intuition customers to upgrade their advanced visualization systems with a custom lung workflow. By leveraging Intuition's capabilities in lung segmentation, volumetric histogram analysis, and automation, physicians quickly receive colorized densities and lung composition values that can be analyzed and quantified with little to no manual editing required. LDA-II is available as a remotely installed upgrade to Intuition customers or comes included as standard functionality with a subscription to TeraRecon’s new AI-ready Intuition Titanium Suite.
Emergency Lung AI Suite
The newly released Emergency Lung AI Suite provides fast access to lung density analysis tools across the enterprise and securely extends access to physicians working remotely. This cloud-based rapid response platform allows physicians to upload cases, anonymize patient data, process the LDA-II algorithm, and receive notification of results regardless of where they are located. Whether in the emergency department, radiology, pulmonology, or at home, this pure cloud offering requires no installation and may be integrated to diagnostic and point-of-care solutions in minutes. The Emergency Lung AI Suite is extensible to other clinical use cases within a health system’s imaging and crisis readiness infrastructure.
For more information: www.terarecon.com


 February 23, 2026
February 23, 2026 









