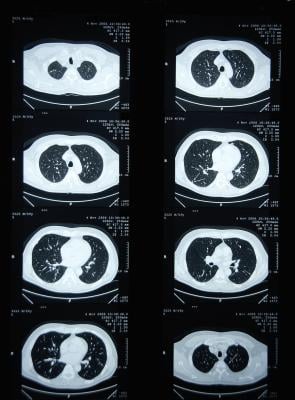
April 27, 2020 — Computed tomography (CT) scans for people at risk for lung cancer lead to earlier diagnoses and improve survival rates, but they can also lead to overtreatment when suspicious nodules turn out to be benign.
A study published in American Journal of Respiratory and Critical Care Medicine indicates that an artificial intelligence strategy can correctly assess and categorize these indeterminate pulmonary nodules (IPNs). When compared to the conventional risk models clinicians currently use, the algorithm developed by the team of researchers in a very large dataset (15,693 nodules) reclassified IPNs into low-risk or high-risk categories in over a third of cancers and benign nodules.
"These results suggest the potential clinical utility of this deep learning algorithm to revise the probability of cancer among IPNs aiming to decrease invasive procedures and shorten time to diagnosis," said Pierre Massion, M.D., Cornelius Vanderbilt Chair in Medicine at Vanderbilt University, the study's lead author.
Currently, clinicians refer to guidelines issued by the American College of Radiology and the American College of Chest Physicians. Adherence to these guidelines can be variable, and how patient cases are classified can be subjective. With the goal of providing clinicians with an unbiased assessment tool, the researchers developed an algorithm based on datasets from the National Lung Screening Trial, Vanderbilt University Medical Center and Oxford University Hospital. Their study is the first to validate a risk stratification tool on multiple independent cohorts and to show reclassification performance that is significantly superior to existing risk models.
With IPNs, clinicians are often faced with the dilemma of weighing whether to advise a patient to undergo an invasive surgical procedure, which may be unnecessary, against a watch and wait strategy, which may result in delaying needed cancer treatment. A definitive diagnosis of an IPN can take up to two years.
Better assessment tools are needed by clinicians as screenings for patients at risk for lung cancer increase. Lung cancer is the leading cause of cancer-related death in the United States and globally. The overall five-year survival rate is 21.7 percent, but it is much greater (92 percent) for those patients who receive an early diagnosis of stage IA1 non-small cell cancer.
For more information: www.


 February 25, 2026
February 25, 2026 









