December 4, 2018 — ScreenPoint Medical has signed a memorandum of understanding (MOU) with Volpara Health Technologies. Volpara will deliver ScreenPoint’s artificial intelligence (AI)-based products to customers as part of its expanding VolparaEnterprise AI cloud ecosystem. Together, the two companies expect to improve the clinical performance of mammography and drive the earlier detection of breast cancer. The partnership will leverage Volpara’s growing footprint in the breast imaging marketplace and will provide an opportunity for ScreenPoint to enter the U.S. market.
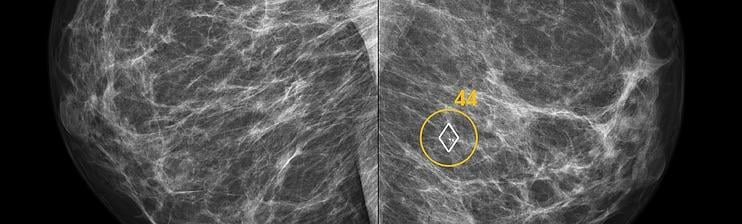
ScreenPoint recently announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Transpara detection and decision support software, designed to assist radiologists with the reading of screening mammograms. Transpara is the first AI application for detecting breast cancer in screening mammograms to gain FDA 510(k) clearance, based on its functionality that interactively provides support for detection and diagnosis.
“Compared to traditional mammography computer-aided detection (CAD) products, ScreenPoint’s decision support product Transpara delivers much more clinically useful information to the radiologist,” said Ritse Mann, M.D., Ph.D., breast imaging specialist at Radboud University Medical Center, Nijmegen, Netherlands, who uses the system clinically and evaluated its performance in several studies, “I can use Transpara as a second reader and its opinion is as good as when I would ask a colleague.”
For more information: www.volparasolutions.com, www.screenpoint-medical.com


 February 06, 2026
February 06, 2026 









