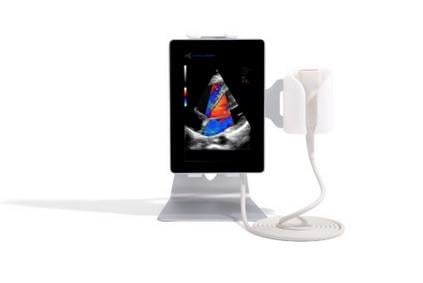
October 18, 2017 — Sonoscanner recently announced it received U.S. Food and Drug Administration (FDA) approval to market its new multipurpose ultrasound device, the U-lite EXP.
Following the U.S. commercialization of the U-lite in 2015, Sonoscanner's second generation ultra-portable ultrasound scanner, the U-Lite EXP, offers an improved diagnostic performance through a better image definition. By proposing probes up to 256 elements 18Mhz, the new system increases the resolution two-fold compared to traditional ultrasound systems. U-Lite EXP's very high-definition probes allows such diagnoses as small mammary tumors or musculoskeletal pain diseases.
With its six high-definition interchangeable probes and its patented probe connector, the U-Lite EXP can perform a wide range of examinations in many specialties: gynecology, obstetrics, urology, anesthesia, endocrinology, osteoarticular and general medicine. Lightweight (less than 600g), with an intuitive touch-screen interface, the device has been designed to meet the challenges of mobility and ease of use.
For more information: www.sonoscanner.com


 February 05, 2026
February 05, 2026 









